Tuesday Poster Session
Category: Liver
P3948 - Leve-TOX-cetam
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
Has Audio

Benjamin Heriford, DO
UT Health San Antonio
San Antonio, TX
Presenting Author(s)
Benjamin Heriford, DO1, Grace Hopp, DO, MBA1, Lisa Pedicone, PhD2, Carmen Landaverde, MD3, Eric Lawitz, MD3, Jan Petrasek, MD, PhD4, Fred Poordad, MD3, Fabian Rodas, MD3, Francis Sharkey, MD1, Eugenia Tsai, MD3
1UT Health San Antonio, San Antonio, TX; 2Texas Liver Institute, San Antonio, TX; 3UT Health San Antonio, Texas Liver Institute, San Antonio, TX; 4UT Health San Antonio, Texas Liver Institute, Jackson, MS
Introduction: Levetiracetam (LEV) is one of the most widely prescribed anti-epileptic medications. We present a case of drug induced liver injury (DILI) from levetiracetam.
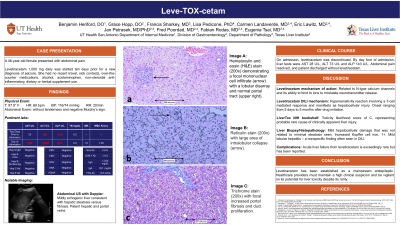
Case Description/Methods: A 46-year-old female with history of seizure and hypertension presented to the hospital with abdominal pain. LEV 1,000 mg daily was started ten days prior for a new diagnosis of seizure. Patient reported no fever, myalgias, respiratory or urinary symptoms, hypotension, or recurrent seizure activity. She reported no recent travel, sick contacts, over-the-counter medications, alcohol, acetaminophen, non-steroidal anti-inflammatory, dietary or herbal supplement use. Laboratory tests revealed AST 728 U/L, ALT 1,089 U/L, ALP 271 U/L, TB 0.5 mg/dL and INR 1.0, increased compared to liver tests two days prior (AST 23 U/L, ALT 48 U/L, ALP 119 U/L, TB 0.2 mg/dL.) Viral and autoimmune workup were negative. Abdominal ultrasound showed mildly echogenic liver with patent vasculature, and normal gallbladder and common bile duct. LEV was discontinued on admission for suspicion of DILI based on Roussel Uclaf Causality Assessment Method (RUCAM) score 12.0. Liver biopsy demonstrated mild lobular hepatitis, minimal steatosis, lobular disarray and intralobular collapse (Figure 1). By day four of admission, liver tests were AST 28 U/L, ALT 73 U/L and ALP 143 U/L.
Discussion: Levetiracetam is a pyrrolidine derivative indicated for the treatment of focal and primary generalized tonic-clonic seizures. The mechanism of levetiracetam is not well understood, but it has been widely used due to its tolerability and rapid efficacy. DILI with levetiracetam is rare because there is minimal hepatic metabolism and does not utilize cytochrome P450. LEV has a toxicity likelihood score of C per LiverTox NIH bookshelf, representing probable rare cause of clinically apparent liver injury. Mechanism of action is a hypersensitivity reaction involving a T-cell mediated response and manifests as hepatocellular injury with onset ranging from 5 days to 5 months after drug initiation. Acute liver failure from LEV is exceedingly rare but has been reported. Histological evaluation typically reveals lobular hepatitis, nonspecific findings as often seen in DILI.
Levetiracetam has been established as a mainstream antiepileptic. Healthcare providers must maintain a high clinical suspicion and be vigilant on its potential for liver toxicity despite its rarity.

Disclosures:
Benjamin Heriford, DO1, Grace Hopp, DO, MBA1, Lisa Pedicone, PhD2, Carmen Landaverde, MD3, Eric Lawitz, MD3, Jan Petrasek, MD, PhD4, Fred Poordad, MD3, Fabian Rodas, MD3, Francis Sharkey, MD1, Eugenia Tsai, MD3. P3948 - Leve-TOX-cetam, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1UT Health San Antonio, San Antonio, TX; 2Texas Liver Institute, San Antonio, TX; 3UT Health San Antonio, Texas Liver Institute, San Antonio, TX; 4UT Health San Antonio, Texas Liver Institute, Jackson, MS
Introduction: Levetiracetam (LEV) is one of the most widely prescribed anti-epileptic medications. We present a case of drug induced liver injury (DILI) from levetiracetam.
Case Description/Methods: A 46-year-old female with history of seizure and hypertension presented to the hospital with abdominal pain. LEV 1,000 mg daily was started ten days prior for a new diagnosis of seizure. Patient reported no fever, myalgias, respiratory or urinary symptoms, hypotension, or recurrent seizure activity. She reported no recent travel, sick contacts, over-the-counter medications, alcohol, acetaminophen, non-steroidal anti-inflammatory, dietary or herbal supplement use. Laboratory tests revealed AST 728 U/L, ALT 1,089 U/L, ALP 271 U/L, TB 0.5 mg/dL and INR 1.0, increased compared to liver tests two days prior (AST 23 U/L, ALT 48 U/L, ALP 119 U/L, TB 0.2 mg/dL.) Viral and autoimmune workup were negative. Abdominal ultrasound showed mildly echogenic liver with patent vasculature, and normal gallbladder and common bile duct. LEV was discontinued on admission for suspicion of DILI based on Roussel Uclaf Causality Assessment Method (RUCAM) score 12.0. Liver biopsy demonstrated mild lobular hepatitis, minimal steatosis, lobular disarray and intralobular collapse (Figure 1). By day four of admission, liver tests were AST 28 U/L, ALT 73 U/L and ALP 143 U/L.
Discussion: Levetiracetam is a pyrrolidine derivative indicated for the treatment of focal and primary generalized tonic-clonic seizures. The mechanism of levetiracetam is not well understood, but it has been widely used due to its tolerability and rapid efficacy. DILI with levetiracetam is rare because there is minimal hepatic metabolism and does not utilize cytochrome P450. LEV has a toxicity likelihood score of C per LiverTox NIH bookshelf, representing probable rare cause of clinically apparent liver injury. Mechanism of action is a hypersensitivity reaction involving a T-cell mediated response and manifests as hepatocellular injury with onset ranging from 5 days to 5 months after drug initiation. Acute liver failure from LEV is exceedingly rare but has been reported. Histological evaluation typically reveals lobular hepatitis, nonspecific findings as often seen in DILI.
Levetiracetam has been established as a mainstream antiepileptic. Healthcare providers must maintain a high clinical suspicion and be vigilant on its potential for liver toxicity despite its rarity.

Figure: Figure 1 (a-c) Histologic findings on liver biopsy consistent with drug induced liver injury from levetiracetam. (a) Hematoxylin and eosin (H&E) stain (200x) demonstrating a focal mononuclear cell infiltrate (arrow) with a lobular disarray and normal portal tract (upper right); (b) Reticulin stain (200x) with large area of intralobular collapse (arrows); (c) Trichrome stain (200x) with focal increased portal fibrosis and duct proliferation.
Disclosures:
Benjamin Heriford indicated no relevant financial relationships.
Grace Hopp indicated no relevant financial relationships.
Lisa Pedicone indicated no relevant financial relationships.
Carmen Landaverde indicated no relevant financial relationships.
Eric Lawitz: 89Bio, Inc – Grant/Research Support. AbbVie – Grant/Research Support, Speakers Bureau. Akero – Advisor or Review Panel Member, Grant/Research Support. Allergan – Grant/Research Support. Alnylam – Grant/Research Support. Amgen – Grant/Research Support. Ascelia Pharma – Grant/Research Support. AstraZeneca – Grant/Research Support. Axcella Health – Grant/Research Support. Boehringer Ingelheim – Advisor or Review Panel Member, Grant/Research Support. Bristol Myers Squibb – Advisor or Review Panel Member, Grant/Research Support. Conatus Pharmaceuticals – Grant/Research Support. Cymabay – Grant/Research Support. CytoDyn – Grant/Research Support. DSM – Grant/Research Support. Durect Corporation – Grant/Research Support. Gilead – Speakers Bureau. Intercept – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Metacrine – Advisor or Review Panel Member. Novo Nordisk – Advisor or Review Panel Member. Sagimet – Advisor or Review Panel Member. Terns – Advisor or Review Panel Member.
Jan Petrasek indicated no relevant financial relationships.
Fred Poordad indicated no relevant financial relationships.
Fabian Rodas indicated no relevant financial relationships.
Francis Sharkey indicated no relevant financial relationships.
Eugenia Tsai indicated no relevant financial relationships.
Benjamin Heriford, DO1, Grace Hopp, DO, MBA1, Lisa Pedicone, PhD2, Carmen Landaverde, MD3, Eric Lawitz, MD3, Jan Petrasek, MD, PhD4, Fred Poordad, MD3, Fabian Rodas, MD3, Francis Sharkey, MD1, Eugenia Tsai, MD3. P3948 - Leve-TOX-cetam, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
