Tuesday Poster Session
Category: IBD
P3608 - Corticosteroid-Free Remission in Anti-TNF-Failed Crohn’s Disease Patients Treated With Vedolizumab as Second-Line Biologic Therapy: A Real World Data Review
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
Has Audio

Christian Agboton, MD
Takeda
Cambridge, MA
Presenting Author(s)
Christian Agboton, MD1, Dirk Lindner, MSc2
1Takeda, Cambridge, MA; 2Takeda, Zurich, Zurich, Switzerland
Introduction: Clinical response to vedolizumab has been reported as being significantly lower in patients exposed to anti-TNFs [1,2]. However, in most countries anti-TNFs remain the de facto first biologic therapies for patients with Crohn’s disease (CD). After failure of a first Anti-TNF drug, change of class of therapy is increasingly recommended in lieu of a second anti-TNF [3]. Using corticosteroid-free (CSF) remission as the main clinical outcome, we collected data on vedolizumab used as second biologic in CD patients who failed anti-TNF therapies.
Methods: PubMed and Embase were searched for real world data from cohorts of adult patients with at least 95% of exposure to anti-TNFs, at least 6 months of data, direct access to patients’ data (no claims database), and CSF remissions (no cumulative data) were selected. Additionally, the use rates of corticosteroids and other concomitant medications at baseline were extracted.
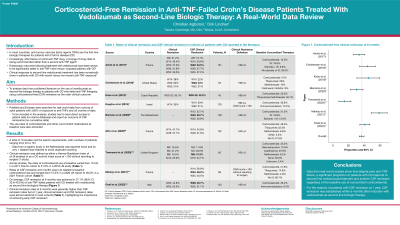
Results: A total of 10 studies met the search requirements, with patient numbers ranging from N=45 to N=161. One author from the Netherlands reported twice on the same registry, only one dataset was retained to avoid counting patients twice. Clinical remission was defined as either a Harvey Bradshaw index of less than or equal to 4 (8 studies) or as a CD activity index score < 150 without resort to surgery (1 study). Across studies, the rates of corticosteroid use at baseline varied from 15.5% in a 2017 French cohort to 51.0% in a 2018 US study. Rates of CSF remission at 6 months were not related to baseline corticosteroid use and ranged from 15.3% in a 2020 UK report to 58.2% in a 2021 French cohort. On average, CSF remission at 6 months was achieved in 37.1%, (95% CI 33.6 - 40.5%) of TNF failed CD patients treated with vedolizumab as second line biological therapy [Table & Figure]. Clinical remission rates at 6 months were generally higher, but at 1 year, clinical remission and CSF remission rates were almost identical in most cohorts, highlighting the importance of achieving early CSF remission.
Discussion: Despite anti-TNF failure, a significant proportion of CD patients may respond to vedolizumab and achieve CSF remission, regardless of the baseline use of concomitant corticosteroids. This benefit is established within 6 months after induction.
References
1. Verstockt, B, et al. 2019. J Crohn’s and Colitis 14:332–41.
2. Gils, A, et al. 2017. Gastro 152:S380.
3. Zhuleku, E, et al. 2022. Therap Adv in Gastro 15:17562848221130554.

Disclosures:
Christian Agboton, MD1, Dirk Lindner, MSc2. P3608 - Corticosteroid-Free Remission in Anti-TNF-Failed Crohn’s Disease Patients Treated With Vedolizumab as Second-Line Biologic Therapy: A Real World Data Review, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Takeda, Cambridge, MA; 2Takeda, Zurich, Zurich, Switzerland
Introduction: Clinical response to vedolizumab has been reported as being significantly lower in patients exposed to anti-TNFs [1,2]. However, in most countries anti-TNFs remain the de facto first biologic therapies for patients with Crohn’s disease (CD). After failure of a first Anti-TNF drug, change of class of therapy is increasingly recommended in lieu of a second anti-TNF [3]. Using corticosteroid-free (CSF) remission as the main clinical outcome, we collected data on vedolizumab used as second biologic in CD patients who failed anti-TNF therapies.
Methods: PubMed and Embase were searched for real world data from cohorts of adult patients with at least 95% of exposure to anti-TNFs, at least 6 months of data, direct access to patients’ data (no claims database), and CSF remissions (no cumulative data) were selected. Additionally, the use rates of corticosteroids and other concomitant medications at baseline were extracted.
Results: A total of 10 studies met the search requirements, with patient numbers ranging from N=45 to N=161. One author from the Netherlands reported twice on the same registry, only one dataset was retained to avoid counting patients twice. Clinical remission was defined as either a Harvey Bradshaw index of less than or equal to 4 (8 studies) or as a CD activity index score < 150 without resort to surgery (1 study). Across studies, the rates of corticosteroid use at baseline varied from 15.5% in a 2017 French cohort to 51.0% in a 2018 US study. Rates of CSF remission at 6 months were not related to baseline corticosteroid use and ranged from 15.3% in a 2020 UK report to 58.2% in a 2021 French cohort. On average, CSF remission at 6 months was achieved in 37.1%, (95% CI 33.6 - 40.5%) of TNF failed CD patients treated with vedolizumab as second line biological therapy [Table & Figure]. Clinical remission rates at 6 months were generally higher, but at 1 year, clinical remission and CSF remission rates were almost identical in most cohorts, highlighting the importance of achieving early CSF remission.
Discussion: Despite anti-TNF failure, a significant proportion of CD patients may respond to vedolizumab and achieve CSF remission, regardless of the baseline use of concomitant corticosteroids. This benefit is established within 6 months after induction.
References
1. Verstockt, B, et al. 2019. J Crohn’s and Colitis 14:332–41.
2. Gils, A, et al. 2017. Gastro 152:S380.
3. Zhuleku, E, et al. 2022. Therap Adv in Gastro 15:17562848221130554.

Figure: Fig: Corticosteroid-free remission after 6 months
Disclosures:
Christian Agboton: Takeda – Employee, Stock Options.
Dirk Lindner: Takeda – Employee, Stock Options.
Christian Agboton, MD1, Dirk Lindner, MSc2. P3608 - Corticosteroid-Free Remission in Anti-TNF-Failed Crohn’s Disease Patients Treated With Vedolizumab as Second-Line Biologic Therapy: A Real World Data Review, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
