Tuesday Poster Session
Category: IBD
P3638 - Characterization of Hospitalized Patients With Ulcerative Colitis Treated With Tofacitinib in the OCTAVE Clinical Program for up to 7.8 Years
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
Has Audio
- CH
Christina Ha, MD
Mayo Clinic Arizona
Scottsdale, AZ
Presenting Author(s)
Carlos Taxonera, 1, Christina Ha, MD2, Maria del Pilar Fortes, 3, Sean Gardiner, 3, Rajiv Mundayat, 3, Subrata Ghosh, 4, Susan J. Connor, 5
1Inflammatory Bowel Disease Unit, Hospital Clínico San Carlos and Instituto de Investigación del Hospital Clínico San Carlos, Madrid, Madrid, Spain; 2Mayo Clinic Arizona, Scottsdale, AZ; 3Pfizer Inc., New York, NY; 4APC Microbiome Ireland, College of Medicine and Health, University College, Cork, Cork, Ireland; 5Liverpool Hospital, University of New South Wales Sydney, Ingham Institute of Applied Medical Research, Sydney, New South Wales, Australia
Introduction: Tofacitinib is an oral small molecule Janus kinase inhibitor for the treatment of ulcerative colitis (UC). The efficacy and safety of tofacitinib were demonstrated in the OCTAVE clinical program.1,2 Here, we present an analysis of the number and causes of UC-related hospitalizations during the OCTAVE clinical program including up to 7.8 years’ tofacitinib exposure.
Methods: This descriptive analysis comprised patients (pts) hospitalized for UC who received placebo or tofacitinib 10 mg twice daily (BID) in OCTAVE Induction 1&2 (NCT01465763; NCT01458951),1 and pts who received placebo, or tofacitinib 5 or 10 mg BID, in OCTAVE Sustain (NCT01458574),1 or ≥ 1 dose of tofacitinib in OCTAVE Open (NCT01470612).2 Demographic and clinical characteristics, proportions, causes, and other aspects of UC-related hospitalizations were evaluated.
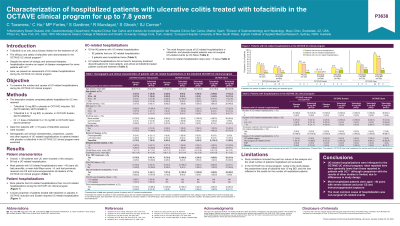
Results: Overall, 1,139 pts with UC were included in this analysis. Demographic and disease characteristics of pts with UC-related hospitalizations were generally similar across treatment groups in each study; most pts were < 40 years of age, had pancolitis, a mean total Mayo score > 8, and prior treatment with oral corticosteroids (CS) and immunosuppressants. Ninety-five pts had UC-related hospitalizations, of whom 92 had one UC-related hospitalization and 3 were hospitalized twice (Table). In OCTAVE Induction 1&2, OCTAVE Sustain, and OCTAVE Open, 2.1%, 1.0%, and 6.6% of pts, respectively, had UC-related hospitalizations with tofacitinib 10 mg BID vs 1.0% and 2.9% of pts receiving tofacitinib 5 mg BID in OCTAVE Sustain and OCTAVE Open, and 3.4% and 4.0% of pts receiving placebo in OCTAVE Induction 1&2 and OCTAVE Sustain, respectively. UC-related hospitalizations did not lead to temporary treatment discontinuations in most pts (Table). The most frequent cause of UC-related hospitalizations in tofacitinib- and placebo-treated pts was non surgical UC-related events (ie UC flares; Table).
Discussion: UC-related hospitalizations were infrequent in the OCTAVE clinical program, and rates were generally lower than those reported in pts with UC treated with biologic therapies.3 Most hospitalized pts were < 40 years of age with severe disease and prior CS and immunosuppressant exposure. The most common cause of hospitalization was non-surgical UC-related events.
References
1. Sandborn et al. N Engl J Med 2017;376:1723–36
2. Sandborn et al. Aliment Pharmacol Ther 2022;55:464–78
3. Bressler et al. J Crohns Colitis 2021;15:1694–1706
Disclosures:
Carlos Taxonera, 1, Christina Ha, MD2, Maria del Pilar Fortes, 3, Sean Gardiner, 3, Rajiv Mundayat, 3, Subrata Ghosh, 4, Susan J. Connor, 5. P3638 - Characterization of Hospitalized Patients With Ulcerative Colitis Treated With Tofacitinib in the OCTAVE Clinical Program for up to 7.8 Years, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Inflammatory Bowel Disease Unit, Hospital Clínico San Carlos and Instituto de Investigación del Hospital Clínico San Carlos, Madrid, Madrid, Spain; 2Mayo Clinic Arizona, Scottsdale, AZ; 3Pfizer Inc., New York, NY; 4APC Microbiome Ireland, College of Medicine and Health, University College, Cork, Cork, Ireland; 5Liverpool Hospital, University of New South Wales Sydney, Ingham Institute of Applied Medical Research, Sydney, New South Wales, Australia
Introduction: Tofacitinib is an oral small molecule Janus kinase inhibitor for the treatment of ulcerative colitis (UC). The efficacy and safety of tofacitinib were demonstrated in the OCTAVE clinical program.1,2 Here, we present an analysis of the number and causes of UC-related hospitalizations during the OCTAVE clinical program including up to 7.8 years’ tofacitinib exposure.
Methods: This descriptive analysis comprised patients (pts) hospitalized for UC who received placebo or tofacitinib 10 mg twice daily (BID) in OCTAVE Induction 1&2 (NCT01465763; NCT01458951),1 and pts who received placebo, or tofacitinib 5 or 10 mg BID, in OCTAVE Sustain (NCT01458574),1 or ≥ 1 dose of tofacitinib in OCTAVE Open (NCT01470612).2 Demographic and clinical characteristics, proportions, causes, and other aspects of UC-related hospitalizations were evaluated.
Results: Overall, 1,139 pts with UC were included in this analysis. Demographic and disease characteristics of pts with UC-related hospitalizations were generally similar across treatment groups in each study; most pts were < 40 years of age, had pancolitis, a mean total Mayo score > 8, and prior treatment with oral corticosteroids (CS) and immunosuppressants. Ninety-five pts had UC-related hospitalizations, of whom 92 had one UC-related hospitalization and 3 were hospitalized twice (Table). In OCTAVE Induction 1&2, OCTAVE Sustain, and OCTAVE Open, 2.1%, 1.0%, and 6.6% of pts, respectively, had UC-related hospitalizations with tofacitinib 10 mg BID vs 1.0% and 2.9% of pts receiving tofacitinib 5 mg BID in OCTAVE Sustain and OCTAVE Open, and 3.4% and 4.0% of pts receiving placebo in OCTAVE Induction 1&2 and OCTAVE Sustain, respectively. UC-related hospitalizations did not lead to temporary treatment discontinuations in most pts (Table). The most frequent cause of UC-related hospitalizations in tofacitinib- and placebo-treated pts was non surgical UC-related events (ie UC flares; Table).
Discussion: UC-related hospitalizations were infrequent in the OCTAVE clinical program, and rates were generally lower than those reported in pts with UC treated with biologic therapies.3 Most hospitalized pts were < 40 years of age with severe disease and prior CS and immunosuppressant exposure. The most common cause of hospitalization was non-surgical UC-related events.
References
1. Sandborn et al. N Engl J Med 2017;376:1723–36
2. Sandborn et al. Aliment Pharmacol Ther 2022;55:464–78
3. Bressler et al. J Crohns Colitis 2021;15:1694–1706
Disclosures:
Carlos Taxonera: AbbVie – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Dr Falk Pharma – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Faes Farma – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Ferring Pharmaceuticals – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Fresenius Kab – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Galapagos – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Janssen – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. MSD – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Pfizer Inc – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Shire Pharmaceuticals – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Takeda – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Tillotts – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau.
Christina Ha: AbbVie – Advisory Committee/Board Member, Consultant, Grant/Research Support. Bristol-Myers Squibb – Advisory Committee/Board Member, Consultant. Helmsley Charitable Trust, – Grant/Research Support. Janssen – Advisory Committee/Board Member, Consultant. Lily – Advisory Committee/Board Member, Consultant. Pfizer Inc – Advisory Committee/Board Member, Consultant, Grant/Research Support. Roivant – Advisory Committee/Board Member, Consultant. Takeda – Advisory Committee/Board Member, Consultant.
Maria del Pilar Fortes: Pfizer Inc – Employee, Shareholder.
Sean Gardiner: Pfizer Inc – Employee, Shareholder.
Rajiv Mundayat: Pfizer Inc – Employee, Shareholder.
Subrata Ghosh: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Bristol-Myers Squibb – Consultant. Celgene – Speakers Bureau. Eli Lilly – Speakers Bureau. Ferring – Speakers Bureau. Galapagos – Speakers Bureau. Gilead – Speakers Bureau. GlaxoSmithKline – Grant/Research Support. Janssen – Consultant, Speakers Bureau. MSD – Speakers Bureau. Novo Nordisk – Consultant. Pfizer – Consultant, Speakers Bureau. Roche – Consultant. Takeda – Consultant, Speakers Bureau.
Susan Connor: AbbVie – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Agency for Clinical Innovation – Grant/Research Support. Amgen – Advisory Committee/Board Member. Bristol-Myers Squibb – Advisory Committee/Board Member, Grant/Research Support, Paid for Registration for ECCO 2023 and Accommodation at ECCO 2023, Speakers Bureau. Celltrion – Advisory Committee/Board Member, Grant/Research Support. Chiesi – Advisory Committee/Board Member, Grant/Research Support. Dr Falk – Grant/Research Support, Speakers Bureau. Eli Lilly – Advisory Committee/Board Member. Ferring – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Fresenius Kabi – Advisory Committee/Board Member. Gastroenterological Society of Australia – Grant/Research Support. GlaxoSmithKlein – Advisory Committee/Board Member. Janssen – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Medical Research Future Fund – Grant/Research Support. MSD – Advisory Committee/Board Member, Grant/Research Support. Novartis – Advisory Committee/Board Member. Organon – Advisory Committee/Board Member. Pfizer Inc – Advisor or Review Panel Member, Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Sandoz – Grant/Research Support, Speakers Bureau. SONIC Pathology – Stock-privately held company. Takeda – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. The Leona M and Harry B Helmsley Charitable Trust – Grant/Research Support.
Carlos Taxonera, 1, Christina Ha, MD2, Maria del Pilar Fortes, 3, Sean Gardiner, 3, Rajiv Mundayat, 3, Subrata Ghosh, 4, Susan J. Connor, 5. P3638 - Characterization of Hospitalized Patients With Ulcerative Colitis Treated With Tofacitinib in the OCTAVE Clinical Program for up to 7.8 Years, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
