Tuesday Poster Session
Category: IBD
P3639 - Intravenous Immunoglobulin for Treatment of Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
Has Audio
.jpg)
Benjamin Gow-Lee, MD
University of Utah School of Medicine
Salt Lake City, UT
Presenting Author(s)
Emre Dal, MD1, Benjamin Gow-Lee, MD2, Amir Kashani, MD, MPH2, John F. Valentine, MD2
1University of Utah, Salt Lake City, UT; 2University of Utah School of Medicine, Salt Lake City, UT
Introduction: The use of intravenous immunoglobulin (IVIg) for the treatment of inflammatory bowel disease (IBD) has been explored in patients with active infections or lack of response to therapeutic agents. However, efficacy data of IVIg in this latter setting are scarce. We conducted a meta-analysis to evaluate the efficacy of IVIg in IBD patients in whom immunosuppressive drugs either failed or were contraindicated.
Methods: A systematic literature search was performed using the keywords “inflammatory bowel disease,” “Crohn’s disease,” “ulcerative colitis,” and “intravenous immunoglobulin” through November 2022. Studies with IBD patients treated with IVIg were included in the analysis. Case reports were excluded. The efficacy of the IVIg therapy was assessed by pooled estimate of clinical remission rate, clinical response rate, and means difference of the Crohn's Disease Activity Index (CDAI).
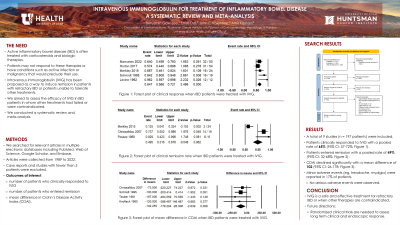
Results: Nine studies met eligibility criteria, including 197 patients: 135 Crohn’s disease (68%), 43 ulcerative colitis (22%), and 19 unclassified (10%). Patients received either single or multiple doses of IVIg at 0.4 g/kg or 10 g/day as monotherapy or in addition to their current treatment regimen. The pooled estimate of clinical response rate (n: 5 studies) following at least one dose of IVIg was 65% (95% CI: 57%-72%) (figure 1A). Among 3 studies, the pooled estimate of clinical remission rate was 49% (95% CI: 32%-68%) (figure 1B). The CDAI score showed a significant decline with a pooled estimate of mean CDAI difference of 102 (95% CI: 26-178) (figure 1C). Minor adverse events (e.g., headache, low-grade fever, myalgia) were reported in 17% of patients. No serious adverse events were observed.
Discussion: This study shows that IVIg is a safe and effective treatment for IBD patients with a lack of response or contraindication to immunosuppressive therapy. Randomized trials are needed to assess long-term clinical and endoscopic response to IVIg in this population.

Disclosures:
Emre Dal, MD1, Benjamin Gow-Lee, MD2, Amir Kashani, MD, MPH2, John F. Valentine, MD2. P3639 - Intravenous Immunoglobulin for Treatment of Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1University of Utah, Salt Lake City, UT; 2University of Utah School of Medicine, Salt Lake City, UT
Introduction: The use of intravenous immunoglobulin (IVIg) for the treatment of inflammatory bowel disease (IBD) has been explored in patients with active infections or lack of response to therapeutic agents. However, efficacy data of IVIg in this latter setting are scarce. We conducted a meta-analysis to evaluate the efficacy of IVIg in IBD patients in whom immunosuppressive drugs either failed or were contraindicated.
Methods: A systematic literature search was performed using the keywords “inflammatory bowel disease,” “Crohn’s disease,” “ulcerative colitis,” and “intravenous immunoglobulin” through November 2022. Studies with IBD patients treated with IVIg were included in the analysis. Case reports were excluded. The efficacy of the IVIg therapy was assessed by pooled estimate of clinical remission rate, clinical response rate, and means difference of the Crohn's Disease Activity Index (CDAI).
Results: Nine studies met eligibility criteria, including 197 patients: 135 Crohn’s disease (68%), 43 ulcerative colitis (22%), and 19 unclassified (10%). Patients received either single or multiple doses of IVIg at 0.4 g/kg or 10 g/day as monotherapy or in addition to their current treatment regimen. The pooled estimate of clinical response rate (n: 5 studies) following at least one dose of IVIg was 65% (95% CI: 57%-72%) (figure 1A). Among 3 studies, the pooled estimate of clinical remission rate was 49% (95% CI: 32%-68%) (figure 1B). The CDAI score showed a significant decline with a pooled estimate of mean CDAI difference of 102 (95% CI: 26-178) (figure 1C). Minor adverse events (e.g., headache, low-grade fever, myalgia) were reported in 17% of patients. No serious adverse events were observed.
Discussion: This study shows that IVIg is a safe and effective treatment for IBD patients with a lack of response or contraindication to immunosuppressive therapy. Randomized trials are needed to assess long-term clinical and endoscopic response to IVIg in this population.

Figure: Forest plots evaluating pooled estimates of A) clinical response, B) remission, and C) difference in mean Crohn's Disease Activity Index (CDAI) when intravenous immunoglobulin (IVIg) is used to treat inflammatory bowel disease (IBD).
Disclosures:
Emre Dal indicated no relevant financial relationships.
Benjamin Gow-Lee indicated no relevant financial relationships.
Amir Kashani indicated no relevant financial relationships.
John Valentine: AbbVie – Grant/Research Support. Abivax – Grant/Research Support. Advanced Molecular Transport – Grant/Research Support. AstraZeneca – Grant/Research Support. Boehringer Ingelheim – Grant/Research Support. Bristol-Myers Squibb – Grant/Research Support. Pfizer – Grant/Research Support. Roche/Genentech – Grant/Research Support. Takeda – Grant/Research Support.
Emre Dal, MD1, Benjamin Gow-Lee, MD2, Amir Kashani, MD, MPH2, John F. Valentine, MD2. P3639 - Intravenous Immunoglobulin for Treatment of Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
