Monday Poster Session
Category: Biliary/Pancreas
P1470 - Itching for Change: The "Therapeutic Gap" in Primary Biliary Cholangitis
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
Has Audio

Marisa-Nicole Zayat, BS
University of Kansas School of Medicine
Wichita, KS
Presenting Author(s)
Antoine Younes, MD1, Marisa-Nicole Zayat, BS1, Micah Vander Griend, MPH, BS1, Hayrettin Okut, PhD1, K. James Kallail, PhD1, Estephan Zayat, MD2
1University of Kansas School of Medicine, Wichita, KS; 2University of Kansas School of Medicine, Kansas Gastroenterology and Kansas Endoscopy, Wichita, KS
Introduction: Obeticholic acid (OCA) is a medication approved for the treatment of primary biliary cholangitis (PBC). It is administered either with ursodeoxycholic acid (UDCA) for patients with an inadequate response to UDCA, or as a monotherapy for adults unable to tolerate UDCA. UDCA had been the first line and only treatment for PBC until the approval of OCA in 2016. Only a few studies presented clinical data on PBC management and long-term follow-up. This study’s aim was to evaluate the long-term response rate of patients on UDCA, the number of patients qualifying for OCA, the number of patients effectively receiving the medication and their clinical outcomes.
Methods: The clinical data of patients with a diagnosis of PBC were reviewed. Patients with concomitant liver comorbidities (e.g. autoimmune hepatitis) as well as patients with incomplete data were excluded. The response rates of patients on UDCA were assessed at 12 to 18 months (short-term), at 24 to 30 months (long-term), and at their last visit according to Paris 1, Paris 2, Barcelona criteria, and the follow-up cut-off alkaline phosphatase (ALP) ≤ 1.67 × upper limits of normal (ULN). We assessed the percentage of patients who were started on OCA and their response rate.
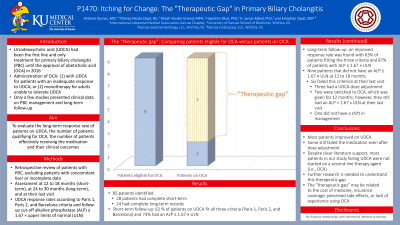
Results: Of the 85 patients identified, 28 patients had complete short-term and 24 had complete long-term records. At the short-term follow-up, 60.71 % of patients on UDCA fit all three criteria (Paris 1, Paris 2, Barcelona) and 73.30% had an ALP ≤ 1.67 × ULN. At the long-term follow-up, an improved response rate was found with 62.50% of patients fitting the three criteria and 87% of patients with ALP ≤ 1.67 × ULN. Of the 9 patients that did not have an ALP ≤ 1.67 × ULN at 12 to 18 months, 6 failed this criterion at their last visit. Notably, three had a UDCA dose adjustment, two were switched to OCA, and one didn’t have a shift in management. Only two patients were switched to OCA which was given for 12 months; however, they still had an ALP > 1.67 x ULN at their last visit.
Discussion: Although most patients improved on UDCA, depending on the criteria used, some still failed the medication even after dose adjustment. Despite clear literature support, most patients in our study failing UDCA were not started on a second-line therapy agent (i.e. OCA). We termed this “the therapeutic gap”. This may be related to the cost of medicine, insurance coverage, perceived side effects, or lack of experience using OCA. Further research is needed to understand this therapeutic gap.

Disclosures:
Antoine Younes, MD1, Marisa-Nicole Zayat, BS1, Micah Vander Griend, MPH, BS1, Hayrettin Okut, PhD1, K. James Kallail, PhD1, Estephan Zayat, MD2. P1470 - Itching for Change: The "Therapeutic Gap" in Primary Biliary Cholangitis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1University of Kansas School of Medicine, Wichita, KS; 2University of Kansas School of Medicine, Kansas Gastroenterology and Kansas Endoscopy, Wichita, KS
Introduction: Obeticholic acid (OCA) is a medication approved for the treatment of primary biliary cholangitis (PBC). It is administered either with ursodeoxycholic acid (UDCA) for patients with an inadequate response to UDCA, or as a monotherapy for adults unable to tolerate UDCA. UDCA had been the first line and only treatment for PBC until the approval of OCA in 2016. Only a few studies presented clinical data on PBC management and long-term follow-up. This study’s aim was to evaluate the long-term response rate of patients on UDCA, the number of patients qualifying for OCA, the number of patients effectively receiving the medication and their clinical outcomes.
Methods: The clinical data of patients with a diagnosis of PBC were reviewed. Patients with concomitant liver comorbidities (e.g. autoimmune hepatitis) as well as patients with incomplete data were excluded. The response rates of patients on UDCA were assessed at 12 to 18 months (short-term), at 24 to 30 months (long-term), and at their last visit according to Paris 1, Paris 2, Barcelona criteria, and the follow-up cut-off alkaline phosphatase (ALP) ≤ 1.67 × upper limits of normal (ULN). We assessed the percentage of patients who were started on OCA and their response rate.
Results: Of the 85 patients identified, 28 patients had complete short-term and 24 had complete long-term records. At the short-term follow-up, 60.71 % of patients on UDCA fit all three criteria (Paris 1, Paris 2, Barcelona) and 73.30% had an ALP ≤ 1.67 × ULN. At the long-term follow-up, an improved response rate was found with 62.50% of patients fitting the three criteria and 87% of patients with ALP ≤ 1.67 × ULN. Of the 9 patients that did not have an ALP ≤ 1.67 × ULN at 12 to 18 months, 6 failed this criterion at their last visit. Notably, three had a UDCA dose adjustment, two were switched to OCA, and one didn’t have a shift in management. Only two patients were switched to OCA which was given for 12 months; however, they still had an ALP > 1.67 x ULN at their last visit.
Discussion: Although most patients improved on UDCA, depending on the criteria used, some still failed the medication even after dose adjustment. Despite clear literature support, most patients in our study failing UDCA were not started on a second-line therapy agent (i.e. OCA). We termed this “the therapeutic gap”. This may be related to the cost of medicine, insurance coverage, perceived side effects, or lack of experience using OCA. Further research is needed to understand this therapeutic gap.

Figure: The “therapeutic gap”: Comparing patients eligible for obeticholic acid (OCA) vs patients on OCA
Disclosures:
Antoine Younes indicated no relevant financial relationships.
Marisa-Nicole Zayat indicated no relevant financial relationships.
Micah Vander Griend indicated no relevant financial relationships.
Hayrettin Okut indicated no relevant financial relationships.
K. James Kallail indicated no relevant financial relationships.
Estephan Zayat indicated no relevant financial relationships.
Antoine Younes, MD1, Marisa-Nicole Zayat, BS1, Micah Vander Griend, MPH, BS1, Hayrettin Okut, PhD1, K. James Kallail, PhD1, Estephan Zayat, MD2. P1470 - Itching for Change: The "Therapeutic Gap" in Primary Biliary Cholangitis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.

