Monday Poster Session
Category: Colon
P1590 - Improvement in Bloating and Overall Complete Spontaneous Bowel Movement Response With Tenapanor Treatment: A Post Hoc Analysis of the IBS-C Clinical Studies
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
Has Audio

Eric Shah, MD, MBA
Geisel School of Medicine
Ann Arbor, MI
Presenting Author(s)
Eric Shah, MD, MBA1, Carol Antequera, DMSc, PA-C2, Dheepika Weerasinghe, PhD3, Yang Yang, PhD3, Susan Edelstein, PhD3
1Geisel School of Medicine, Hanover, NH; 2University of Miami, Miami, FL; 3Ardelyx, Inc., Waltham, MA
Introduction: Tenapanor (TEN) is a first-in-class, minimally systemic inhibitor of intestinal sodium-hydrogen exchanger isoform 3 (NHE3) approved by the FDA for adults with irritable bowel syndrome with constipation (IBS-C). Through NHE3 inhibition, TEN reduces the absorption of sodium leading to softer stool consistency and faster transit. In nonclinical studies, TEN has been shown to also decrease both intestinal permeability and visceral hypersensitivity. The phase 2b (NCT01923428) and phase 3 T3MPO-1 (NCT02621892) and T3MPO-2 (NCT02686138) studies showed that patients treated with TEN 50 mg bid experienced both significant increase in complete spontaneous bowel movements (CSBMs) and decrease in abdominal pain compared to those receiving placebo. The most common adverse event seen across TEN studies was diarrhea.
Bloating is a bothersome abdominal symptom in IBS-C and is a common reason to seek treatment. We previously demonstrated that TEN improves bloating along with other abdominal symptoms in IBS-C. We conducted a post hoc analysis of pooled data from phase 2b and 3 studies to assess the relationship between improvement in bloating and overall CSBM response to TEN treatment.
Methods: Study methods have been described previously. Briefly, the studies enrolled adults with IBS-C who met the Rome III criteria. Patients were randomized to TEN 50 mg bid or placebo bid for 12 or 26 weeks. A phone diary was used to collect data on daily abdominal bloating on a 0 to 10-point scale. For this analysis, TEN-treated patients were categorized into those that met and those that did not meet the overall CSBM response utilizing the regulatory definition of an increase of ≥1 weekly CSBM from baseline for 6/12 weeks. We assessed mean change from baseline in average weekly bloating score stratified on CSBM responder status.
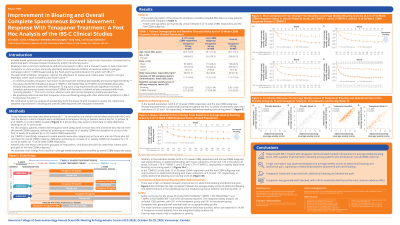
Results: The pooled population of the phase 2b and phase 3 studies included 684 intent-to-treat patients who received TEN. Patient demographics were generally similar across the studies. At week 12, both CSBM Responders and Non-responders had improvements in abdominal bloating with 3.53 and 1.60 score reductions, respectively (Figure).
Discussion: Patients with IBS-C treated with TEN demonstrated marked improvement in average weekly bloating score, with a greater improvement in bloating among patients who achieved an overall CSBM response.

Disclosures:
Eric Shah, MD, MBA1, Carol Antequera, DMSc, PA-C2, Dheepika Weerasinghe, PhD3, Yang Yang, PhD3, Susan Edelstein, PhD3. P1590 - Improvement in Bloating and Overall Complete Spontaneous Bowel Movement Response With Tenapanor Treatment: A Post Hoc Analysis of the IBS-C Clinical Studies, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Geisel School of Medicine, Hanover, NH; 2University of Miami, Miami, FL; 3Ardelyx, Inc., Waltham, MA
Introduction: Tenapanor (TEN) is a first-in-class, minimally systemic inhibitor of intestinal sodium-hydrogen exchanger isoform 3 (NHE3) approved by the FDA for adults with irritable bowel syndrome with constipation (IBS-C). Through NHE3 inhibition, TEN reduces the absorption of sodium leading to softer stool consistency and faster transit. In nonclinical studies, TEN has been shown to also decrease both intestinal permeability and visceral hypersensitivity. The phase 2b (NCT01923428) and phase 3 T3MPO-1 (NCT02621892) and T3MPO-2 (NCT02686138) studies showed that patients treated with TEN 50 mg bid experienced both significant increase in complete spontaneous bowel movements (CSBMs) and decrease in abdominal pain compared to those receiving placebo. The most common adverse event seen across TEN studies was diarrhea.
Bloating is a bothersome abdominal symptom in IBS-C and is a common reason to seek treatment. We previously demonstrated that TEN improves bloating along with other abdominal symptoms in IBS-C. We conducted a post hoc analysis of pooled data from phase 2b and 3 studies to assess the relationship between improvement in bloating and overall CSBM response to TEN treatment.
Methods: Study methods have been described previously. Briefly, the studies enrolled adults with IBS-C who met the Rome III criteria. Patients were randomized to TEN 50 mg bid or placebo bid for 12 or 26 weeks. A phone diary was used to collect data on daily abdominal bloating on a 0 to 10-point scale. For this analysis, TEN-treated patients were categorized into those that met and those that did not meet the overall CSBM response utilizing the regulatory definition of an increase of ≥1 weekly CSBM from baseline for 6/12 weeks. We assessed mean change from baseline in average weekly bloating score stratified on CSBM responder status.
Results: The pooled population of the phase 2b and phase 3 studies included 684 intent-to-treat patients who received TEN. Patient demographics were generally similar across the studies. At week 12, both CSBM Responders and Non-responders had improvements in abdominal bloating with 3.53 and 1.60 score reductions, respectively (Figure).
Discussion: Patients with IBS-C treated with TEN demonstrated marked improvement in average weekly bloating score, with a greater improvement in bloating among patients who achieved an overall CSBM response.

Figure: Figure. Mean (±Standard Error) Change from Baseline in Average Weekly Bloating Score by Overall (6/12 Weeks) CSBM Response Status
Disclosures:
Eric Shah: Ardelyx, Inc. – Advisory Committee/Board Member, Consultant. GI Supply – Advisory Committee/Board Member. Mahana – Consultant. Mylan – Advisory Committee/Board Member. Salix – Advisory Committee/Board Member. Sanofi – Advisory Committee/Board Member, Consultant. Takeda – Consultant.
Carol Antequera indicated no relevant financial relationships.
Dheepika Weerasinghe: Ardelyx, Inc. – Employee, Stock Options.
Yang Yang: Ardelyx, Inc. – Employee, Stock Options.
Susan Edelstein: Ardelyx, Inc. – Employee, Stock Options.
Eric Shah, MD, MBA1, Carol Antequera, DMSc, PA-C2, Dheepika Weerasinghe, PhD3, Yang Yang, PhD3, Susan Edelstein, PhD3. P1590 - Improvement in Bloating and Overall Complete Spontaneous Bowel Movement Response With Tenapanor Treatment: A Post Hoc Analysis of the IBS-C Clinical Studies, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
