Monday Poster Session
Category: Colon
P1592 - Efficacy and Safety of Fecal Microbiota Transplant for First or Second Clostridioides difficile Infection: A Meta-Analysis of Randomized Controlled Trials
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
Has Audio

Sophia Dar, MD
Northshore-Long Island Jewish Medical Center
Springfield , IL
Presenting Author(s)
Abia Shahid, MBBS1, Huzaifa Ahmad Cheema, MBBS1, Ahmad Nawaz, MBBS1, Muhammad Abdullah Ilyas, MBBS1, Farwa Athar, MBBS1, Biah Mustafa, MBBS1, Sophia Haroon Dar, MD2, Rehmat Ullah Awan, MD3
1King Edward Medical University, Lahore, Punjab, Pakistan; 2Northshore-Long Island Jewish Medical Center, New York, NY; 3Ochsner Rush Medical Center, Meridian, MS
Introduction: Fecal microbiota transplantation (FMT) is a proven treatment option for recurrent Clostridioides difficile infection (CDI). However, its efficacy and safety for first or second CDI are unknown. We undertook this meta-analysis to address this knowledge gap by aggregating data from randomized controlled trials (RCTs).
Methods: We searched PubMed, Embase, the Cochrane Library, and ClinicalTrials.gov from inception till January 2023 for all RCTs comparing FMT in addition to medical therapy (MT) versus MT alone in the first or second episode of CDI. We excluded studies that evaluated patients with recurrent CDI (three or more episodes). Our outcomes of interest were: (1) the clinical cure rate at the longest follow-up; (2) all-cause mortality at the longest follow-up; and serious adverse events (SAEs). We used Stata 17.0 to pool risk ratios (RRs) with 95% confidence intervals (CIs) under a random-effects model. We employed the empirical Bayes variance estimator which gives more reliable results when the number of studies is low. We stratified our results according to the MT given to patients (metronidazole vs. vancomycin).
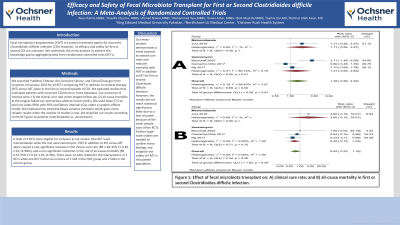
Results: A total of 4 RCTs were eligible for inclusion in our review. One RCT used metronidazole while the rest used vancomycin. FMT in addition to MT versus MT alone caused a non-significant increase in the clinical cure rate (RR 1.38, 95% CI: 0.82-2.32; I2=69%), and a non-significant reduction in the risk of all-cause mortality (RR 0.52, 95% CI: 0.23-1.16; I2=0%). There were no SAEs related to the interventions in 3 RCTs while one RCT had an occurrence of 1 SAE in the FMT group and 2 SAEs in the control group.
Discussion: Our meta-analysis demonstrates a trend towards increased cure rates and reduced mortality with FMT in addition to MT for first or second Clostridium difficile infection. However, the results did not reach statistical significance, likely due to a lack of power because of the small sample sizes of the RCTs. Further large-scale studies are needed to confirm these findings and establish the safety of FMT in this patient population.

Disclosures:
Abia Shahid, MBBS1, Huzaifa Ahmad Cheema, MBBS1, Ahmad Nawaz, MBBS1, Muhammad Abdullah Ilyas, MBBS1, Farwa Athar, MBBS1, Biah Mustafa, MBBS1, Sophia Haroon Dar, MD2, Rehmat Ullah Awan, MD3. P1592 - Efficacy and Safety of Fecal Microbiota Transplant for First or Second Clostridioides difficile Infection: A Meta-Analysis of Randomized Controlled Trials, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1King Edward Medical University, Lahore, Punjab, Pakistan; 2Northshore-Long Island Jewish Medical Center, New York, NY; 3Ochsner Rush Medical Center, Meridian, MS
Introduction: Fecal microbiota transplantation (FMT) is a proven treatment option for recurrent Clostridioides difficile infection (CDI). However, its efficacy and safety for first or second CDI are unknown. We undertook this meta-analysis to address this knowledge gap by aggregating data from randomized controlled trials (RCTs).
Methods: We searched PubMed, Embase, the Cochrane Library, and ClinicalTrials.gov from inception till January 2023 for all RCTs comparing FMT in addition to medical therapy (MT) versus MT alone in the first or second episode of CDI. We excluded studies that evaluated patients with recurrent CDI (three or more episodes). Our outcomes of interest were: (1) the clinical cure rate at the longest follow-up; (2) all-cause mortality at the longest follow-up; and serious adverse events (SAEs). We used Stata 17.0 to pool risk ratios (RRs) with 95% confidence intervals (CIs) under a random-effects model. We employed the empirical Bayes variance estimator which gives more reliable results when the number of studies is low. We stratified our results according to the MT given to patients (metronidazole vs. vancomycin).
Results: A total of 4 RCTs were eligible for inclusion in our review. One RCT used metronidazole while the rest used vancomycin. FMT in addition to MT versus MT alone caused a non-significant increase in the clinical cure rate (RR 1.38, 95% CI: 0.82-2.32; I2=69%), and a non-significant reduction in the risk of all-cause mortality (RR 0.52, 95% CI: 0.23-1.16; I2=0%). There were no SAEs related to the interventions in 3 RCTs while one RCT had an occurrence of 1 SAE in the FMT group and 2 SAEs in the control group.
Discussion: Our meta-analysis demonstrates a trend towards increased cure rates and reduced mortality with FMT in addition to MT for first or second Clostridium difficile infection. However, the results did not reach statistical significance, likely due to a lack of power because of the small sample sizes of the RCTs. Further large-scale studies are needed to confirm these findings and establish the safety of FMT in this patient population.

Figure: Figure 1: Effect of fecal microbiota transplant on: A) clinical cure rate; and B) all-cause mortality in first or second Clostridioides difficile infection.
Disclosures:
Abia Shahid indicated no relevant financial relationships.
Huzaifa Ahmad Cheema indicated no relevant financial relationships.
Ahmad Nawaz indicated no relevant financial relationships.
Muhammad Abdullah Ilyas indicated no relevant financial relationships.
Farwa Athar indicated no relevant financial relationships.
Biah Mustafa indicated no relevant financial relationships.
Sophia Haroon Dar indicated no relevant financial relationships.
Rehmat Ullah Awan indicated no relevant financial relationships.
Abia Shahid, MBBS1, Huzaifa Ahmad Cheema, MBBS1, Ahmad Nawaz, MBBS1, Muhammad Abdullah Ilyas, MBBS1, Farwa Athar, MBBS1, Biah Mustafa, MBBS1, Sophia Haroon Dar, MD2, Rehmat Ullah Awan, MD3. P1592 - Efficacy and Safety of Fecal Microbiota Transplant for First or Second Clostridioides difficile Infection: A Meta-Analysis of Randomized Controlled Trials, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
