Monday Poster Session
Category: Colon
P1616 - Gastrointestinal Toxicities of Proteasome Inhibitor Therapy
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
Has Audio

Samanthika S. Devalaraju, MD
Baylor College of Medicine
Houston, TX
Presenting Author(s)
Jay S. Shah, MD1, Samanthika S. Devalaraju, MD1, Elliot Baerman, MD1, Malek Shatila, MD2, Antonio Pizuorno Machado, MD3, Christine Catinis, MD, MS4, Anusha Thomas, MD2, Yinghong Wang, MD2
1Baylor College of Medicine, Houston, TX; 2University of Texas MD Anderson Cancer Center, Houston, TX; 3University of Texas Health Science Center, Houston, TX; 4University of Texas Health Science Center at Houston, Houston, TX
Introduction: Proteasome inhibitors (PI) are a class of cancer drugs that cause cell death via the accumulation of tumor suppressors and pro-apoptotic proteins. The development of PI, such as Bortezomib, Carfilzomib, and Ixazomib, has provided a breakthrough in the management of many malignancies. A limiting factor in the use of these drugs is their side effect profile, which includes neuropathy, fatigue, and gastrointestinal (GI) toxicity. Despite this, PI remain an integral component of treatment regimens due to improvement in patient outcomes. In this study, we evaluated PI related GI toxicities and compared the differences in GI toxicities between Bortezomib, Carfilzomib, and Ixazomib.
Methods: We conducted a retrospective study among cancer patients at a tertiary care cancer center to investigate the clinical characteristics of PI related GI toxicity. We included patients receiving a PI and excluded patients with GI symptoms from other etiologies.
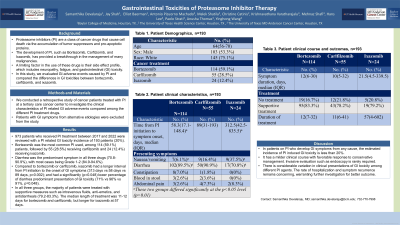
Results: 973 patients who received PI treatment between 2017 and 2022 were reviewed with a PI related GI toxicity incidence of 193 patients (20%). 114 (59%) of the patients received Bortezomib, 55 (29%) received Carfilzomib, and 24 (12%) received Ixazomib. The median time from PI initiation to GI toxicity was 79 days. The most common symptom was diarrhea, present in 169 (88%). The median symptom duration was 12 days. 70 (36%) required hospitalization. 157 (81%) received treatment for their GI toxicity and 99.3% of these patients were treated with supportive care including antimotility agents, antiemetics, and intravenous fluids. 17 patients (8.8%) received colonoscopy at the time of toxicity. 11 (65%) had normal findings and 6 (36%) had evidence of inflammation. 8 patients had calprotectin levels checked and all were within normal range. 145 (92%) patients who received treatment achieved remission. 71 (37%) developed recurrence of GI toxicity. Median time between PI initiation and development of GI toxicity was significantly (p=0.002) higher for Ixazomib (313 days) compared to Bortezomib (58 days) or Carfilzomib (89 days). Ixazomib also had a significantly (p=0.048) lower percentage of GI toxicity presenting as diarrhea (71%) compared to Bortezomib (96%) or Carfilzomib (91%).
Discussion: PI related GI toxicities vary based on different regimens, but overall has a high incidence. However, our review demonstrates that the vast majority of GI toxicities resolved with supportive treatment and had favorable outcome without long term consequence.
Disclosures:
Jay S. Shah, MD1, Samanthika S. Devalaraju, MD1, Elliot Baerman, MD1, Malek Shatila, MD2, Antonio Pizuorno Machado, MD3, Christine Catinis, MD, MS4, Anusha Thomas, MD2, Yinghong Wang, MD2. P1616 - Gastrointestinal Toxicities of Proteasome Inhibitor Therapy, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Baylor College of Medicine, Houston, TX; 2University of Texas MD Anderson Cancer Center, Houston, TX; 3University of Texas Health Science Center, Houston, TX; 4University of Texas Health Science Center at Houston, Houston, TX
Introduction: Proteasome inhibitors (PI) are a class of cancer drugs that cause cell death via the accumulation of tumor suppressors and pro-apoptotic proteins. The development of PI, such as Bortezomib, Carfilzomib, and Ixazomib, has provided a breakthrough in the management of many malignancies. A limiting factor in the use of these drugs is their side effect profile, which includes neuropathy, fatigue, and gastrointestinal (GI) toxicity. Despite this, PI remain an integral component of treatment regimens due to improvement in patient outcomes. In this study, we evaluated PI related GI toxicities and compared the differences in GI toxicities between Bortezomib, Carfilzomib, and Ixazomib.
Methods: We conducted a retrospective study among cancer patients at a tertiary care cancer center to investigate the clinical characteristics of PI related GI toxicity. We included patients receiving a PI and excluded patients with GI symptoms from other etiologies.
Results: 973 patients who received PI treatment between 2017 and 2022 were reviewed with a PI related GI toxicity incidence of 193 patients (20%). 114 (59%) of the patients received Bortezomib, 55 (29%) received Carfilzomib, and 24 (12%) received Ixazomib. The median time from PI initiation to GI toxicity was 79 days. The most common symptom was diarrhea, present in 169 (88%). The median symptom duration was 12 days. 70 (36%) required hospitalization. 157 (81%) received treatment for their GI toxicity and 99.3% of these patients were treated with supportive care including antimotility agents, antiemetics, and intravenous fluids. 17 patients (8.8%) received colonoscopy at the time of toxicity. 11 (65%) had normal findings and 6 (36%) had evidence of inflammation. 8 patients had calprotectin levels checked and all were within normal range. 145 (92%) patients who received treatment achieved remission. 71 (37%) developed recurrence of GI toxicity. Median time between PI initiation and development of GI toxicity was significantly (p=0.002) higher for Ixazomib (313 days) compared to Bortezomib (58 days) or Carfilzomib (89 days). Ixazomib also had a significantly (p=0.048) lower percentage of GI toxicity presenting as diarrhea (71%) compared to Bortezomib (96%) or Carfilzomib (91%).
Discussion: PI related GI toxicities vary based on different regimens, but overall has a high incidence. However, our review demonstrates that the vast majority of GI toxicities resolved with supportive treatment and had favorable outcome without long term consequence.
Disclosures:
Jay Shah indicated no relevant financial relationships.
Samanthika Devalaraju indicated no relevant financial relationships.
Elliot Baerman indicated no relevant financial relationships.
Malek Shatila indicated no relevant financial relationships.
Antonio Pizuorno Machado indicated no relevant financial relationships.
Christine Catinis indicated no relevant financial relationships.
Anusha Thomas indicated no relevant financial relationships.
Yinghong Wang: ilyapharma – Consultant. IOTA – Consultant. Janssen – Consultant. MabQuest – Advisory Committee/Board Member. Sorriso – Consultant. Tillotts – Consultant.
Jay S. Shah, MD1, Samanthika S. Devalaraju, MD1, Elliot Baerman, MD1, Malek Shatila, MD2, Antonio Pizuorno Machado, MD3, Christine Catinis, MD, MS4, Anusha Thomas, MD2, Yinghong Wang, MD2. P1616 - Gastrointestinal Toxicities of Proteasome Inhibitor Therapy, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
