Monday Poster Session
Category: Colorectal Cancer Prevention
P1782 - Utilization Rates of Colonic Preparations in the US – A Large Database Analysis
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
Has Audio

Daniel L. Halberg, PhD
Sebela Pharmaceuticals
Braintree, MA
Presenting Author(s)
Audrey H.. Calderwood, MD, MS1, Eric D. Shah, MD, MBA, FACG2, Daniel L. Halberg, PhD3
1Dartmouth Health, Lebanon, NH; 2University of Michigan, Ann Arbor, MI; 3Sebela Pharmaceuticals, Braintree, MA
Introduction: Colonoscopy is widely recognized as the gold-standard for colorectal cancer screening and the primary diagnostic tool for many diseases of the colon and rectum. Hesitancy around bowel preparation is the number one barrier for patients in scheduling and completing both initial and follow-up colonoscopy. Volume, palatability, and tolerability of the prep are the key factors for patient completion. Modern low volume (LV) preps (≤2L) improve patient experience while preserving cleansing efficacy, with success rates >90%. It is unclear how often LV prep options are being prescribed in comparison to other types of preps, such as high volume (HV) 4L and non-FDA approved over the counter (OTC) preparations. The aim of our study was to evaluate utilization of LV preps in comparison to HV and OTC preps across the US using a large, national prescription dataset.
Methods: This analysis was constructed using inputs from IQVIA Medical Claims, CMS Medicare Part B claims, and Xponent® prescription (Rx) datasets, for the period extending from 2021-Q1 through 2022-Q4. Xponent® is a national Rx dataset, collected from retail, mail order and long-term care channels with a 93% Rx capture rate. Data were obtained by cross referencing National Provider Identifiers (NPI) with procedure claim data based on submitted CPT codes. To avoid double counting, analysis was based on actual patient encounters rather than procedure counts. OTC use was then imputed from unmatched claims and Rx counts.
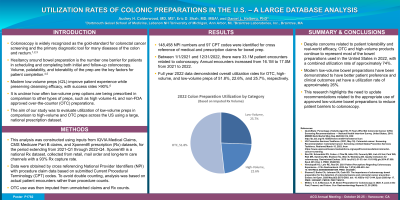
Results: 148,458 NPI numbers and 97 CPT codes were identified for cross reference of medical and prescription claims for bowel prep. Between 1/1/2021 and 12/31/2022, there were 33.1M patient encounters related to colonoscopy. Annual encounters increased from 16.1M to 17.0M from 2021 to 2022. Full year 2022 data demonstrated overall utilization rates for OTC, HV, and LV of 51.8%, 22.6%, and 25.7%, respectively.
Discussion: Despite concerns related to patient tolerability and real-world efficacy, OTC and HV products continue to represent most of the bowel preparations used in the United States in 2022, with a combined utilization rate of approximately 74%. Modern LV bowel preparations have been demonstrated to have better patient preference and clinical outcomes yet have a utilization rate of approximately 26%. This research highlights the need to update recommendations related to the appropriate use of approved LV bowel preparations to reduce patient barriers to colonoscopy.

Disclosures:
Audrey H.. Calderwood, MD, MS1, Eric D. Shah, MD, MBA, FACG2, Daniel L. Halberg, PhD3. P1782 - Utilization Rates of Colonic Preparations in the US – A Large Database Analysis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Dartmouth Health, Lebanon, NH; 2University of Michigan, Ann Arbor, MI; 3Sebela Pharmaceuticals, Braintree, MA
Introduction: Colonoscopy is widely recognized as the gold-standard for colorectal cancer screening and the primary diagnostic tool for many diseases of the colon and rectum. Hesitancy around bowel preparation is the number one barrier for patients in scheduling and completing both initial and follow-up colonoscopy. Volume, palatability, and tolerability of the prep are the key factors for patient completion. Modern low volume (LV) preps (≤2L) improve patient experience while preserving cleansing efficacy, with success rates >90%. It is unclear how often LV prep options are being prescribed in comparison to other types of preps, such as high volume (HV) 4L and non-FDA approved over the counter (OTC) preparations. The aim of our study was to evaluate utilization of LV preps in comparison to HV and OTC preps across the US using a large, national prescription dataset.
Methods: This analysis was constructed using inputs from IQVIA Medical Claims, CMS Medicare Part B claims, and Xponent® prescription (Rx) datasets, for the period extending from 2021-Q1 through 2022-Q4. Xponent® is a national Rx dataset, collected from retail, mail order and long-term care channels with a 93% Rx capture rate. Data were obtained by cross referencing National Provider Identifiers (NPI) with procedure claim data based on submitted CPT codes. To avoid double counting, analysis was based on actual patient encounters rather than procedure counts. OTC use was then imputed from unmatched claims and Rx counts.
Results: 148,458 NPI numbers and 97 CPT codes were identified for cross reference of medical and prescription claims for bowel prep. Between 1/1/2021 and 12/31/2022, there were 33.1M patient encounters related to colonoscopy. Annual encounters increased from 16.1M to 17.0M from 2021 to 2022. Full year 2022 data demonstrated overall utilization rates for OTC, HV, and LV of 51.8%, 22.6%, and 25.7%, respectively.
Discussion: Despite concerns related to patient tolerability and real-world efficacy, OTC and HV products continue to represent most of the bowel preparations used in the United States in 2022, with a combined utilization rate of approximately 74%. Modern LV bowel preparations have been demonstrated to have better patient preference and clinical outcomes yet have a utilization rate of approximately 26%. This research highlights the need to update recommendations related to the appropriate use of approved LV bowel preparations to reduce patient barriers to colonoscopy.

Figure: 2022 Colon Preparation Utilization by Category
Disclosures:
Audrey Calderwood: Dark Canyon Laboratories – Advisory Committee/Board Member.
Eric Shah: Ardelyx – Advisory Committee/Board Member, Consultant. GI Supply/Laborie – Advisory Committee/Board Member, Consultant. Mahana – Consultant. Neuraxis – Consultant. Salix – Advisory Committee/Board Member. Sanofi – Advisory Committee/Board Member, Consultant. Takeda – Consultant.
Daniel Halberg: Sebela Pharmaceuticals – Employee.
Audrey H.. Calderwood, MD, MS1, Eric D. Shah, MD, MBA, FACG2, Daniel L. Halberg, PhD3. P1782 - Utilization Rates of Colonic Preparations in the US – A Large Database Analysis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
