Sunday Poster Session
Category: Colon
P0174 - Identifying Challenges to Harmonizing C. difficile Infection Care Among Specialties: A Critical Step as the Management Toolkit Advances
Sunday, October 22, 2023
3:30 PM - 7:00 PM PT
Location: Exhibit Hall

Jessica R. Allegretti, MD, MPH
Brigham and Women's Hospital, Harvard Medical School
Boston, MA
Presenting Author(s)
Jessica R.. Allegretti, MD, MPH1, Abhishek Deshpande, MD, PhD2, Elizabeth Hohmann, MD3, Nick Tomeo, PhD4
1Brigham and Women's Hospital, Harvard Medical School, Boston, MA; 2Cleveland Clinic, Cleveland, OH; 3Massachusetts General Hospital, Boston, MA; 4American Gastroenterological Association, Bethesda, MD
Introduction: Impacting nearly half a million Americans annually, Clostridioides difficile infection (CDI) is associated with a high burden of hospitalizations, morbidity, and mortality. There remains several factors that contribute to CDI diagnosis and management challenges. Harmonizing care among specialties is even more critical now as live biotherapeutic products (LBP) become available to prevent recurrent CDI.
Methods: In Feb.-Mar. 2023, a nationally representative online survey was fielded to gastroenterologists (GI), infectious disease specialists (ID), and primary care physicians (PCP). Respondents were limited to those who treat a minimum of three (GI/ID) or one (PCP) CDI patients/month. Survey questions included self-reported diagnostic and treatment practices, knowledge/use of clinical guidelines, and expectations for the use of LBPs. Data were analyzed using column proportions test.
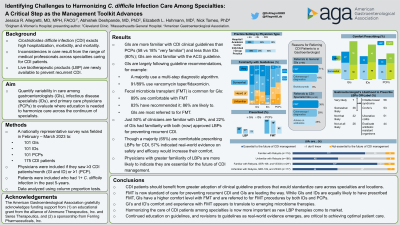
Results: Complete responses were received from 101 GIs, 101 IDs, and 100 PCPs. GIs included 61 in individual or group practices and 39 in hospital or academic centers. Most GIs are familiar with current clinical guidelines for CDI (97% very or somewhat knowledgeable). The GIs in the sample largely follow guideline-recommendations for treatment of initial CDI, with 91% having recommended a vancomycin taper and 89% fidaxomicin in the past, and few (16%) describing metronidazole as effective. Fecal microbiota transplant (FMT) has become standard care for preventing CDI recurrence with 89% of GIs comfortable (very or somewhat) prescribing FMT, 83% having recommended FMT to a patient, and 86% likely to prescribe FMT in the future. In line with this, the most common rationale for IDs or PCPs to refer CDI patients to GIs is for FMT. GIs also note that they are less familiar with the recently approved LBPs for the prevention of CDI recurrence, with just 50% familiar with the first LBP approved for use in 2022 and 22% familiar with the LBP approved in 2023. Though a majority (65%) stated they are very or somewhat comfortable prescribing them. When asked what would increase comfort in prescribing LBPs for CDI, GIs (57%) indicated interest in more real-world evidence on safety and efficacy.
Discussion: Harmonizing care of patients with CDI among specialties is critical in light of the recent approval of new LBPs and more agents coming. Continued education on guidelines as well as real world evidence is critical to achieving this.
Disclosures:
Jessica R.. Allegretti, MD, MPH1, Abhishek Deshpande, MD, PhD2, Elizabeth Hohmann, MD3, Nick Tomeo, PhD4. P0174 - Identifying Challenges to Harmonizing C. difficile Infection Care Among Specialties: A Critical Step as the Management Toolkit Advances, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Brigham and Women's Hospital, Harvard Medical School, Boston, MA; 2Cleveland Clinic, Cleveland, OH; 3Massachusetts General Hospital, Boston, MA; 4American Gastroenterological Association, Bethesda, MD
Introduction: Impacting nearly half a million Americans annually, Clostridioides difficile infection (CDI) is associated with a high burden of hospitalizations, morbidity, and mortality. There remains several factors that contribute to CDI diagnosis and management challenges. Harmonizing care among specialties is even more critical now as live biotherapeutic products (LBP) become available to prevent recurrent CDI.
Methods: In Feb.-Mar. 2023, a nationally representative online survey was fielded to gastroenterologists (GI), infectious disease specialists (ID), and primary care physicians (PCP). Respondents were limited to those who treat a minimum of three (GI/ID) or one (PCP) CDI patients/month. Survey questions included self-reported diagnostic and treatment practices, knowledge/use of clinical guidelines, and expectations for the use of LBPs. Data were analyzed using column proportions test.
Results: Complete responses were received from 101 GIs, 101 IDs, and 100 PCPs. GIs included 61 in individual or group practices and 39 in hospital or academic centers. Most GIs are familiar with current clinical guidelines for CDI (97% very or somewhat knowledgeable). The GIs in the sample largely follow guideline-recommendations for treatment of initial CDI, with 91% having recommended a vancomycin taper and 89% fidaxomicin in the past, and few (16%) describing metronidazole as effective. Fecal microbiota transplant (FMT) has become standard care for preventing CDI recurrence with 89% of GIs comfortable (very or somewhat) prescribing FMT, 83% having recommended FMT to a patient, and 86% likely to prescribe FMT in the future. In line with this, the most common rationale for IDs or PCPs to refer CDI patients to GIs is for FMT. GIs also note that they are less familiar with the recently approved LBPs for the prevention of CDI recurrence, with just 50% familiar with the first LBP approved for use in 2022 and 22% familiar with the LBP approved in 2023. Though a majority (65%) stated they are very or somewhat comfortable prescribing them. When asked what would increase comfort in prescribing LBPs for CDI, GIs (57%) indicated interest in more real-world evidence on safety and efficacy.
Discussion: Harmonizing care of patients with CDI among specialties is critical in light of the recent approval of new LBPs and more agents coming. Continued education on guidelines as well as real world evidence is critical to achieving this.
Disclosures:
Jessica Allegretti: Abbvie – Consultant, Speakers Bureau. Adiso Therapeutics – Consultant. Artizan – Consultant. Artugen Therapeutics – Consultant. Baccain – Consultant. Bristol-Myers Squibb/Celgene – Consultant, Speakers Bureau. Ferring Pharmaceuticals – Consultant. Finch Therapeutics – Consultant. Iterative Scopes – Consultant. Janssen – Consultant, Grant/Research Support, Speakers Bureau. Merck – Consultant, Grant/Research Support. Morphic – Consultant. Pandion Therapeutics – Consultant. Pfizer – Consultant, Grant/Research Support. Roivant Sciences – Consultant. Seres Therapeutics – Consultant. Servatus – Consultant. Summit – Consultant.
Abhishek Deshpande: Clorox Company – Grant/Research Support. Seres Therapeutics – Grant/Research Support.
Elizabeth Hohmann: Gilead Sciences – Consultant. Kowa Pharmaceuticals – Consultant. Seres Therapeutics – Grant/Research Support.
Nick Tomeo: Bristol Myers Squibb – Grant/Research Support. Ferring Pharmaceuticals – Grant/Research Support. Seres / Aimmune Therapeutics – Grant/Research Support.
Jessica R.. Allegretti, MD, MPH1, Abhishek Deshpande, MD, PhD2, Elizabeth Hohmann, MD3, Nick Tomeo, PhD4. P0174 - Identifying Challenges to Harmonizing C. difficile Infection Care Among Specialties: A Critical Step as the Management Toolkit Advances, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
