Sunday Poster Session
Category: IBD
P0714 - Major Discordance - Gastroenterologist Survey on IBD Medication Authorization Denials
Sunday, October 22, 2023
3:30 PM - 7:00 PM PT
Location: Exhibit Hall
Has Audio

Anastasia Naritsin, DO
Lahey Hospital and Medical Center
Woburn, MA
Presenting Author(s)
Anastasia Naritsin, DO1, Neev Mehta, MD2, Randall Pellish, MD2
1Lahey Hospital and Medical Center, Burlington, MA; 2Lahey Hospital, Burlington, MA
Introduction: Insurance prior authorizations are commonly required for biologics and small-molecule drugs to treat inflammatory bowel disease (IBD). Authorization denials appear to occur in a wide variety of scenarios, including denials of standard and non-standard dosing directed by accepted clinical indications. We performed a cross-sectional survey on a broad variety of specific clinical scenarios to assess experience and opinions on whether or not the authorization denials are in accordance with clinical expertise.
Methods: A national cross-sectional survey was distributed via email and social media to over 600 practicing gastroenterologists between May and September 2022. The survey consisted of 23 scenarios where authorization denials occur with two thematic domains including general experiences with authorization denial scenarios and opinions on clinical appropriateness of the authorization denials. Redcap data management system was utilized for data collection and analysis.
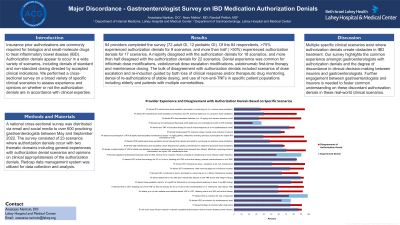
Results: 84 providers completed the survey (72 adult GI, 12 pediatric GI). Of the 84 respondents, >75% experienced authorization denials for 9 scenarios, and more than half ( >50%) experienced authorization denials for 17 scenarios. A majority disagreed with the authorization denials for 18 scenarios, and more than half disagreed with the authorization denials for 22 scenarios. Denial experience was common for infliximab dose modifications, vedolizumab dose escalation modifications, ustekinumab first-time therapy and maintenance dosing. The bulk of disagreement with authorization denials included scenarios of dose escalation and re-induction guided by both loss of clinical response and/or therapeutic drug monitoring, denial of re-authorizations of stable dosing, and use of non-anti-TNFs in specific patient populations including elderly and patients with multiple comorbidities.
Discussion: Multiple specific clinical scenarios exist where authorization denials create obstacles in IBD treatment. Our survey highlights the common experience amongst gastroenterologists with authorization denials and the degree of discordance in clinical decision-making between insurers and gastroenterologists. Further engagement between gastroenterologists and insurers is needed to foster common understanding on these discordant authorization denials in these real-world clinical scenarios.

Disclosures:
Anastasia Naritsin, DO1, Neev Mehta, MD2, Randall Pellish, MD2. P0714 - Major Discordance - Gastroenterologist Survey on IBD Medication Authorization Denials, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Lahey Hospital and Medical Center, Burlington, MA; 2Lahey Hospital, Burlington, MA
Introduction: Insurance prior authorizations are commonly required for biologics and small-molecule drugs to treat inflammatory bowel disease (IBD). Authorization denials appear to occur in a wide variety of scenarios, including denials of standard and non-standard dosing directed by accepted clinical indications. We performed a cross-sectional survey on a broad variety of specific clinical scenarios to assess experience and opinions on whether or not the authorization denials are in accordance with clinical expertise.
Methods: A national cross-sectional survey was distributed via email and social media to over 600 practicing gastroenterologists between May and September 2022. The survey consisted of 23 scenarios where authorization denials occur with two thematic domains including general experiences with authorization denial scenarios and opinions on clinical appropriateness of the authorization denials. Redcap data management system was utilized for data collection and analysis.
Results: 84 providers completed the survey (72 adult GI, 12 pediatric GI). Of the 84 respondents, >75% experienced authorization denials for 9 scenarios, and more than half ( >50%) experienced authorization denials for 17 scenarios. A majority disagreed with the authorization denials for 18 scenarios, and more than half disagreed with the authorization denials for 22 scenarios. Denial experience was common for infliximab dose modifications, vedolizumab dose escalation modifications, ustekinumab first-time therapy and maintenance dosing. The bulk of disagreement with authorization denials included scenarios of dose escalation and re-induction guided by both loss of clinical response and/or therapeutic drug monitoring, denial of re-authorizations of stable dosing, and use of non-anti-TNFs in specific patient populations including elderly and patients with multiple comorbidities.
Discussion: Multiple specific clinical scenarios exist where authorization denials create obstacles in IBD treatment. Our survey highlights the common experience amongst gastroenterologists with authorization denials and the degree of discordance in clinical decision-making between insurers and gastroenterologists. Further engagement between gastroenterologists and insurers is needed to foster common understanding on these discordant authorization denials in these real-world clinical scenarios.

Figure: Title: Provider Experience and Disagreement with Authorization Denials Based on Specific Scenarios
X-Axis: Percent
X-Axis: Percent
Disclosures:
Anastasia Naritsin indicated no relevant financial relationships.
Neev Mehta indicated no relevant financial relationships.
Randall Pellish indicated no relevant financial relationships.
Anastasia Naritsin, DO1, Neev Mehta, MD2, Randall Pellish, MD2. P0714 - Major Discordance - Gastroenterologist Survey on IBD Medication Authorization Denials, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
