Sunday Poster Session
Category: IBD
P0787 - A Promising Novel Therapeutic Strategy for Inflammatory Bowel Disease Through Enhanced Efferocytosis and Anti-Inflammatory Mechanisms
Sunday, October 22, 2023
3:30 PM - 7:00 PM PT
Location: Exhibit Hall
Has Audio
- MK
Minkyoung Kim, PhD
NEXEL Co., Ltd.
Gangseo-gu, Seoul-t'ukpyolsi, Republic of Korea
Presenting Author(s)
Joon-Chul Kim, PhD, Soeun Jeong, MS, Jaehun Lee, PhD, Gukdo Kim, MS, Jinjoo Oh, MS, Jung-Hyuck Park, PhD, Dong-Hun Woo, MS, Choongseong Han, PhD, Minkyoung Kim, PhD
NEXEL Co., Ltd., Gangseo-gu, Seoul-t'ukpyolsi, Republic of Korea
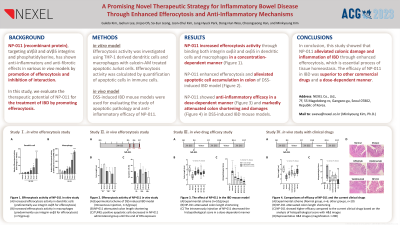
Introduction: Inflammatory bowel disease (IBD) continues to present significant challenges in treatment. Prolonged utilization of existing therapies frequently results in insufficient outcomes, dependency, serious adverse effects, or recurrent disease exacerbations, underlining the urgency for new, innovative treatment strategies. This study explores the potential of a novel drug candidate, NP-011 (C2-deleted MFG-E8), aimed at IBD management through a unique mode of action, with promising therapeutic effectiveness and potential advantages over existing treatment options.
Methods: Our hypothesis was that NP-011 functions as a bridging molecule, facilitating the process of efferocytosis. We initially measured the binding affinity of NP-011 to phosphatidylserine (PS) through a radioligand binding assay. Following this, we examined the absorption of fluorescent PS microparticles and apoptotic cells (ACs) by macrophages, and subsequently observed alterations in the expression of HO-1 post absorption of ACs. We then used a DSS-induced UC mouse model to assess the in vivo efficacy of NP-011, focusing on its impact on weight regain, restoration of colon length, reduction of inflammation, and mitigation of necrosis.
Results:
NP-011 demonstrated strong affinity to phosphatidylserine (PS) and boosted the uptake of PS and apoptotic cells (ACs) by macrophages, leading to an enhanced expression of HO-1. The maximum count of TUNEL-positive cells in mouse colons was observed on day five post-exposure to DSS. By strategically administering NP-011 on this pivotal day, we managed to effectively reduce the count of ACs, and lower the concentration of inflammation and necrosis markers in the blood. This resulted in a significant improvement in histopathological assessments. Importantly, NP-011 exceeded the efficacy of its predecessor protein and currently available clinical biologics.
Discussion: NP-011's novel mechanism of action holds potential in addressing the unmet needs in ulcerative colitis treatment. This potential stems from its capacity to facilitate efferocytosis, promoting the cessation of inflammation and steering the cytokine profile to a state of reduced inflammation. Moreover, NP-011 contributes to the repair and reorganization of tissues, staves off the emergence of autoimmunity, and assists in maintaining the balance of the immune system.
Disclosures:
Joon-Chul Kim, PhD, Soeun Jeong, MS, Jaehun Lee, PhD, Gukdo Kim, MS, Jinjoo Oh, MS, Jung-Hyuck Park, PhD, Dong-Hun Woo, MS, Choongseong Han, PhD, Minkyoung Kim, PhD. P0787 - A Promising Novel Therapeutic Strategy for Inflammatory Bowel Disease Through Enhanced Efferocytosis and Anti-Inflammatory Mechanisms, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
NEXEL Co., Ltd., Gangseo-gu, Seoul-t'ukpyolsi, Republic of Korea
Introduction: Inflammatory bowel disease (IBD) continues to present significant challenges in treatment. Prolonged utilization of existing therapies frequently results in insufficient outcomes, dependency, serious adverse effects, or recurrent disease exacerbations, underlining the urgency for new, innovative treatment strategies. This study explores the potential of a novel drug candidate, NP-011 (C2-deleted MFG-E8), aimed at IBD management through a unique mode of action, with promising therapeutic effectiveness and potential advantages over existing treatment options.
Methods: Our hypothesis was that NP-011 functions as a bridging molecule, facilitating the process of efferocytosis. We initially measured the binding affinity of NP-011 to phosphatidylserine (PS) through a radioligand binding assay. Following this, we examined the absorption of fluorescent PS microparticles and apoptotic cells (ACs) by macrophages, and subsequently observed alterations in the expression of HO-1 post absorption of ACs. We then used a DSS-induced UC mouse model to assess the in vivo efficacy of NP-011, focusing on its impact on weight regain, restoration of colon length, reduction of inflammation, and mitigation of necrosis.
Results:
NP-011 demonstrated strong affinity to phosphatidylserine (PS) and boosted the uptake of PS and apoptotic cells (ACs) by macrophages, leading to an enhanced expression of HO-1. The maximum count of TUNEL-positive cells in mouse colons was observed on day five post-exposure to DSS. By strategically administering NP-011 on this pivotal day, we managed to effectively reduce the count of ACs, and lower the concentration of inflammation and necrosis markers in the blood. This resulted in a significant improvement in histopathological assessments. Importantly, NP-011 exceeded the efficacy of its predecessor protein and currently available clinical biologics.
Discussion: NP-011's novel mechanism of action holds potential in addressing the unmet needs in ulcerative colitis treatment. This potential stems from its capacity to facilitate efferocytosis, promoting the cessation of inflammation and steering the cytokine profile to a state of reduced inflammation. Moreover, NP-011 contributes to the repair and reorganization of tissues, staves off the emergence of autoimmunity, and assists in maintaining the balance of the immune system.
Disclosures:
Joon-Chul Kim indicated no relevant financial relationships.
Soeun Jeong indicated no relevant financial relationships.
Jaehun Lee indicated no relevant financial relationships.
Gukdo Kim indicated no relevant financial relationships.
Jinjoo Oh indicated no relevant financial relationships.
Jung-Hyuck Park indicated no relevant financial relationships.
Dong-Hun Woo indicated no relevant financial relationships.
Choongseong Han indicated no relevant financial relationships.
Minkyoung Kim indicated no relevant financial relationships.
Joon-Chul Kim, PhD, Soeun Jeong, MS, Jaehun Lee, PhD, Gukdo Kim, MS, Jinjoo Oh, MS, Jung-Hyuck Park, PhD, Dong-Hun Woo, MS, Choongseong Han, PhD, Minkyoung Kim, PhD. P0787 - A Promising Novel Therapeutic Strategy for Inflammatory Bowel Disease Through Enhanced Efferocytosis and Anti-Inflammatory Mechanisms, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
