Sunday Poster Session
Category: IBD
P0793 - JAK(1i) to the Rescue! Upadacitinib as Rescue Therapy in Acute Severe Ulcerative Colitis
Sunday, October 22, 2023
3:30 PM - 7:00 PM PT
Location: Exhibit Hall
Has Audio
.jpg)
Adam Madej, DO
Banner Health
Tucson, AZ
Presenting Author(s)
Adam Madej, DO1, Amar Vedamurthy, MD1, Sasha Taleban, MD2
1Banner Health, Tucson, AZ; 2University of Arizona College of Medicine, Tucson, AZ
Introduction: Acute severe ulcerative colitis (ASUC) is a life-threatening event that complicates the course of 25% of ulcerative colitis (UC) patients. Upadacitinib (UPA) is a JAK1 inhibitor approved for use in moderate-severe UC. In the outpatient setting, UPA has been shown to induce clinical remission in 34% and 52% of UC patients after 8 and 52 weeks of treatment, respectively. UPA appears to work quickly with rectal bleeding and stool frequency resolution by week 2 and as early as day 1. However, the role of UPA in ASUC remains unclear.
Case Description/Methods: A 27-year-old female diagnosed with UC in 2018 and developed avascular necrosis in both knees after exposure to corticosteroids. She had previously failed treatment with vedolizumab and combination therapy of 10 mg/kg every 4 weeks of infliximab and azathioprine. She began the process of starting UPA, but before it was initiated, the patient’s symptoms progressed, and she was admitted to the hospital.
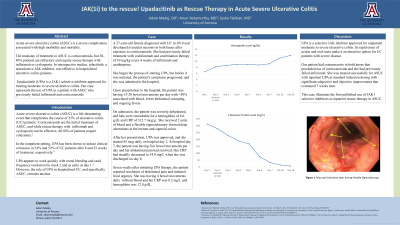
Upon presentation to the hospital, the patient was having 15-20 bowel movements per day with < 50% associated with blood, lower abdominal cramping, and ongoing fevers. On admission, the patient was severely dehydrated, and labs were remarkable for a hemoglobin of 6.6 g/dL and CRP of 312.7 mcg/g. She received 2 units of blood and a flexible sigmoidoscopy showed deep ulcerations in the rectum and sigmoid colon. After her presentation, UPA was approved, and she started 45 mcg daily on hospital day 2. At hospital day 5, the patient was having five bowel movements per day and her abdominal pain had resolved. Her CRP had steadily decreased to 34.0 mg/L when she was discharged on day 8. Four weeks after initiating UPA therapy, the patient reported resolution of abdominal pain and minimal fecal urgency. She was having 4 bowel movements daily without blood and her CRP was 8.2 mg/L and HgB was 12.0 g/dL.
Discussion: UPA is a selective JAK inhibitor approved for outpatient moderate to severe ulcerative colitis. Its rapid onset of action and oral route make it an attractive option for UC patients with severe disease. Our patient had osteonecrosis in both knees that precluded use of corticosteroids and she had previously failed infliximab. She was treated successfully for ASUC with inpatient UPA at standard induction dosing with significant subjective and objective improvement that continued 4 weeks later. This case illustrates the first published use of JAK1 selective inhibitors as inpatient rescue therapy in ASUC.
Disclosures:
Adam Madej, DO1, Amar Vedamurthy, MD1, Sasha Taleban, MD2. P0793 - JAK(1i) to the Rescue! Upadacitinib as Rescue Therapy in Acute Severe Ulcerative Colitis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Banner Health, Tucson, AZ; 2University of Arizona College of Medicine, Tucson, AZ
Introduction: Acute severe ulcerative colitis (ASUC) is a life-threatening event that complicates the course of 25% of ulcerative colitis (UC) patients. Upadacitinib (UPA) is a JAK1 inhibitor approved for use in moderate-severe UC. In the outpatient setting, UPA has been shown to induce clinical remission in 34% and 52% of UC patients after 8 and 52 weeks of treatment, respectively. UPA appears to work quickly with rectal bleeding and stool frequency resolution by week 2 and as early as day 1. However, the role of UPA in ASUC remains unclear.
Case Description/Methods: A 27-year-old female diagnosed with UC in 2018 and developed avascular necrosis in both knees after exposure to corticosteroids. She had previously failed treatment with vedolizumab and combination therapy of 10 mg/kg every 4 weeks of infliximab and azathioprine. She began the process of starting UPA, but before it was initiated, the patient’s symptoms progressed, and she was admitted to the hospital.
Upon presentation to the hospital, the patient was having 15-20 bowel movements per day with < 50% associated with blood, lower abdominal cramping, and ongoing fevers. On admission, the patient was severely dehydrated, and labs were remarkable for a hemoglobin of 6.6 g/dL and CRP of 312.7 mcg/g. She received 2 units of blood and a flexible sigmoidoscopy showed deep ulcerations in the rectum and sigmoid colon. After her presentation, UPA was approved, and she started 45 mcg daily on hospital day 2. At hospital day 5, the patient was having five bowel movements per day and her abdominal pain had resolved. Her CRP had steadily decreased to 34.0 mg/L when she was discharged on day 8. Four weeks after initiating UPA therapy, the patient reported resolution of abdominal pain and minimal fecal urgency. She was having 4 bowel movements daily without blood and her CRP was 8.2 mg/L and HgB was 12.0 g/dL.
Discussion: UPA is a selective JAK inhibitor approved for outpatient moderate to severe ulcerative colitis. Its rapid onset of action and oral route make it an attractive option for UC patients with severe disease. Our patient had osteonecrosis in both knees that precluded use of corticosteroids and she had previously failed infliximab. She was treated successfully for ASUC with inpatient UPA at standard induction dosing with significant subjective and objective improvement that continued 4 weeks later. This case illustrates the first published use of JAK1 selective inhibitors as inpatient rescue therapy in ASUC.
Disclosures:
Adam Madej indicated no relevant financial relationships.
Amar Vedamurthy indicated no relevant financial relationships.
Sasha Taleban indicated no relevant financial relationships.
Adam Madej, DO1, Amar Vedamurthy, MD1, Sasha Taleban, MD2. P0793 - JAK(1i) to the Rescue! Upadacitinib as Rescue Therapy in Acute Severe Ulcerative Colitis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
