Sunday Poster Session
Category: Interventional Endoscopy
P0890 - Novel Use of Tandem Stenting to Create Continuous Patent Channel for Management of Gastric Outlet Obstruction Caused by Diffuse Gastric Adenocarcinoma
Sunday, October 22, 2023
3:30 PM - 7:00 PM PT
Location: Exhibit Hall
Has Audio

Anusha Singhania, MBBS
Swedish Medical Center
Seattle, WA
Presenting Author(s)
Anusha Singhania, MBBS1, Tavishi Girotra, MBBS1, Shreya Chopra, MBBS1, Christopher J.. Carlson, MD1, Rajnish Mishra, MD1, Mohit Girotra, MD2
1Swedish Medical Center, Seattle, WA; 2Swedish Medical Center, Washington State University Elson S. Floyd School of Medicine, Seattle, WA
Introduction: Gastric outlet obstruction (GOO) is a commonly reported sequela of advanced upper GI malignancies (gastric/duodenal/pancreatic), with management approaches often aimed at symptom palliation. Endoscopic stenting (ES) is routinely offered to patients with limited life expectancy or poor performance status.
We report a unique case of diffuse gastric adenocarcinomatous infiltration extending from gastro-esophageal junction (GEJ) to pylorus, which precluded bridging with single stent or other interventional options like bypass gastro-jejunostomy (GJ), therefore managed using 2 stents, placed in tandem, with satisfactory functional outcome.
Case Description/Methods: A 46-year-old lady diagnosed 2 years prior with metastatic gastric adenocarcinoma with bilateral ovarian metastases (likely Krukenberg tumor), on Eliquis for recent acute right internal jugular thrombosis, presented with subacute onset of epigastric pain, and persistent nausea/vomiting, suspicious of GOO. She denied any fever, jaundice but endorsed mild dysphagia and stomach fullness.
Esophagogastroduodenoscopy (EGD) revealed fibrotic stenosis of distal esophagus with widespread tumor involvement starting at GEJ, which circumferentially involved gastric cardia, corpus, antrum and pyloric channel, with slight luminal widening in the mid-corpus. Rescue ES was decided for palliative relief. However, due to the long involvement, it was not amenable to a single stent. Hence, tandem stenting was done using distal 25 x 90 mm and proximal 25 x 120 mm uncovered metallic stents, thereby creating a continuous patent channel from the GEJ to the duodenal bulb. Subsequently, she was able to tolerate mechanically altered diet.
Discussion: Patients with malignant GOO usually have dismal prognosis (3-6 months median survival), and focus is on palliation by establishing oral intake by restoring GI continuity, for which GJ and ES are safe and effective modalities, with similar clinical success and adverse event rates.
In our case, extensive tumor infiltration from GEJ to the pylorus made GJ unviable. Moreover, in patients with an expected survival of less than 2-6 months, as in ours, ES is preferred due to its less invasive nature, rapid relief and patient preference. Therefore, due to the extensive tumor involvement, a novel approach of tandem placement of 2 self-expandable metal stents was undertaken, to create a continuous path from the GEJ to duodenal bulb, thereby bypassing the entire carcinomatous stomach, allowing food passage and symptom palliation.

Disclosures:
Anusha Singhania, MBBS1, Tavishi Girotra, MBBS1, Shreya Chopra, MBBS1, Christopher J.. Carlson, MD1, Rajnish Mishra, MD1, Mohit Girotra, MD2. P0890 - Novel Use of Tandem Stenting to Create Continuous Patent Channel for Management of Gastric Outlet Obstruction Caused by Diffuse Gastric Adenocarcinoma, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Swedish Medical Center, Seattle, WA; 2Swedish Medical Center, Washington State University Elson S. Floyd School of Medicine, Seattle, WA
Introduction: Gastric outlet obstruction (GOO) is a commonly reported sequela of advanced upper GI malignancies (gastric/duodenal/pancreatic), with management approaches often aimed at symptom palliation. Endoscopic stenting (ES) is routinely offered to patients with limited life expectancy or poor performance status.
We report a unique case of diffuse gastric adenocarcinomatous infiltration extending from gastro-esophageal junction (GEJ) to pylorus, which precluded bridging with single stent or other interventional options like bypass gastro-jejunostomy (GJ), therefore managed using 2 stents, placed in tandem, with satisfactory functional outcome.
Case Description/Methods: A 46-year-old lady diagnosed 2 years prior with metastatic gastric adenocarcinoma with bilateral ovarian metastases (likely Krukenberg tumor), on Eliquis for recent acute right internal jugular thrombosis, presented with subacute onset of epigastric pain, and persistent nausea/vomiting, suspicious of GOO. She denied any fever, jaundice but endorsed mild dysphagia and stomach fullness.
Esophagogastroduodenoscopy (EGD) revealed fibrotic stenosis of distal esophagus with widespread tumor involvement starting at GEJ, which circumferentially involved gastric cardia, corpus, antrum and pyloric channel, with slight luminal widening in the mid-corpus. Rescue ES was decided for palliative relief. However, due to the long involvement, it was not amenable to a single stent. Hence, tandem stenting was done using distal 25 x 90 mm and proximal 25 x 120 mm uncovered metallic stents, thereby creating a continuous patent channel from the GEJ to the duodenal bulb. Subsequently, she was able to tolerate mechanically altered diet.
Discussion: Patients with malignant GOO usually have dismal prognosis (3-6 months median survival), and focus is on palliation by establishing oral intake by restoring GI continuity, for which GJ and ES are safe and effective modalities, with similar clinical success and adverse event rates.
In our case, extensive tumor infiltration from GEJ to the pylorus made GJ unviable. Moreover, in patients with an expected survival of less than 2-6 months, as in ours, ES is preferred due to its less invasive nature, rapid relief and patient preference. Therefore, due to the extensive tumor involvement, a novel approach of tandem placement of 2 self-expandable metal stents was undertaken, to create a continuous path from the GEJ to duodenal bulb, thereby bypassing the entire carcinomatous stomach, allowing food passage and symptom palliation.

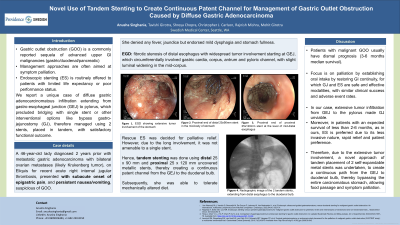
Figure: A. EGD showing extensive tumor involvement of the stomach.
B. EGD showing proximal end of distal 25 x 90 mm stent in the mid-body of stomach.
C. EGD showing proximal end of proximal 25 x 120 mm stent at the level of mid-distal esophagus.
D. Radiographic image of the 2 tandem stents, extending from distal esophagus to the duodenal bulb.
B. EGD showing proximal end of distal 25 x 90 mm stent in the mid-body of stomach.
C. EGD showing proximal end of proximal 25 x 120 mm stent at the level of mid-distal esophagus.
D. Radiographic image of the 2 tandem stents, extending from distal esophagus to the duodenal bulb.
Disclosures:
Anusha Singhania indicated no relevant financial relationships.
Tavishi Girotra indicated no relevant financial relationships.
Shreya Chopra indicated no relevant financial relationships.
Christopher Carlson indicated no relevant financial relationships.
Rajnish Mishra indicated no relevant financial relationships.
Mohit Girotra indicated no relevant financial relationships.
Anusha Singhania, MBBS1, Tavishi Girotra, MBBS1, Shreya Chopra, MBBS1, Christopher J.. Carlson, MD1, Rajnish Mishra, MD1, Mohit Girotra, MD2. P0890 - Novel Use of Tandem Stenting to Create Continuous Patent Channel for Management of Gastric Outlet Obstruction Caused by Diffuse Gastric Adenocarcinoma, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.

