Sunday Poster Session
Category: Liver
P1014 - Severe, Protracted Cholestatic Liver Injury Due to Novel Fat Burner Albutarex V2 in an Active Duty Sailor
Sunday, October 22, 2023
3:30 PM - 7:00 PM PT
Location: Exhibit Hall
Has Audio
- JL
Jessica L. Lilley, MD
Naval Medical Center
San Diego, CA
Presenting Author(s)
Jessica L. Lilley, MD, Maunoo Lee, MD, Brett Sadowski, MD
Naval Medical Center, San Diego, CA
Introduction: Herbal and dietary supplement (HDS) use is rising in the United States, in part due to the use of pharmacologic therapy aimed at weight loss. HDS account for 15-20% of drug induced liver injuries (DILI) according to the Drug-Induced Liver Injury Network (DILIN). In addition, the insufficient regulation from Food and Drug Administration (FDA) regarding supplements pose greater risk as more dietary products are readily available in retail stores. We present a novel case of severe cholestatic DILI from a novel fat burner supplement, Albutarex V2 along with its protracted clinical course.
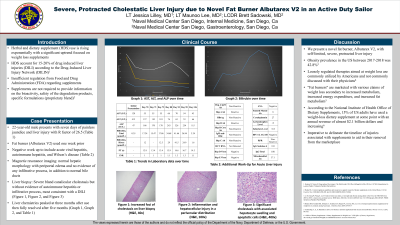
Case Description/Methods: A 22-year-old otherwise healthy male presented with seven days of painless jaundice and pruritus found to have a severe mixed but predominantly cholestatic liver injury with an R factor of 26.5 (initial labs of AST 128 U/L, ALT 415 U/L, alkaline phosphatase 47 IU/L, and bilirubin 0.35 µmol/L) in the setting of fat burner supplementation use (Albutarex V2) one week prior. Three months after supplementation use, the patient’s labs peaked with an R factor 0.6. A broad work up for alternative causes of liver injury including acute viral hepatitis, autoimmune hepatitis, and acute Wilson disease was negative. Magnetic resonance imaging showed normal hepatic morphology with periportal edema and no evidence of any infiltrative process, in addition to normal bile ducts. Given progressive injury, liver biopsy was obtained which revealed severe bland canalicular cholestasis but without evidence of autoimmune hepatitis nor infiltrative process, most consistent with a DILI. The patient’s liver chemistries were monitored to full resolution five months after supplementation use.
Discussion: With steadily increasing obesity rates and exponentially increasing dietary supplement use, loosely regulated therapies aimed at weight loss are more commonly utilized. Weight loss supplements or "fat burners" are typically marketed with claims of increasing energy expenditure by increasing the resting metabolic rate to lead to weight loss. Recognition of the potential adverse effects of these therapies is critical for clinicians since identification and cessation of the offending agent is the most important step in management. This case demonstrates the danger of easily accessible “fat burners” and delineates the protracted and prolonged nature of the cholestatic liver injury associated with the particular supplement, Albutarex V2.

Disclosures:
Jessica L. Lilley, MD, Maunoo Lee, MD, Brett Sadowski, MD. P1014 - Severe, Protracted Cholestatic Liver Injury Due to Novel Fat Burner Albutarex V2 in an Active Duty Sailor, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
Naval Medical Center, San Diego, CA
Introduction: Herbal and dietary supplement (HDS) use is rising in the United States, in part due to the use of pharmacologic therapy aimed at weight loss. HDS account for 15-20% of drug induced liver injuries (DILI) according to the Drug-Induced Liver Injury Network (DILIN). In addition, the insufficient regulation from Food and Drug Administration (FDA) regarding supplements pose greater risk as more dietary products are readily available in retail stores. We present a novel case of severe cholestatic DILI from a novel fat burner supplement, Albutarex V2 along with its protracted clinical course.
Case Description/Methods: A 22-year-old otherwise healthy male presented with seven days of painless jaundice and pruritus found to have a severe mixed but predominantly cholestatic liver injury with an R factor of 26.5 (initial labs of AST 128 U/L, ALT 415 U/L, alkaline phosphatase 47 IU/L, and bilirubin 0.35 µmol/L) in the setting of fat burner supplementation use (Albutarex V2) one week prior. Three months after supplementation use, the patient’s labs peaked with an R factor 0.6. A broad work up for alternative causes of liver injury including acute viral hepatitis, autoimmune hepatitis, and acute Wilson disease was negative. Magnetic resonance imaging showed normal hepatic morphology with periportal edema and no evidence of any infiltrative process, in addition to normal bile ducts. Given progressive injury, liver biopsy was obtained which revealed severe bland canalicular cholestasis but without evidence of autoimmune hepatitis nor infiltrative process, most consistent with a DILI. The patient’s liver chemistries were monitored to full resolution five months after supplementation use.
Discussion: With steadily increasing obesity rates and exponentially increasing dietary supplement use, loosely regulated therapies aimed at weight loss are more commonly utilized. Weight loss supplements or "fat burners" are typically marketed with claims of increasing energy expenditure by increasing the resting metabolic rate to lead to weight loss. Recognition of the potential adverse effects of these therapies is critical for clinicians since identification and cessation of the offending agent is the most important step in management. This case demonstrates the danger of easily accessible “fat burners” and delineates the protracted and prolonged nature of the cholestatic liver injury associated with the particular supplement, Albutarex V2.

Figure: Legend:
Orange line: ALT (alanine transaminase) in U/L
Blue line: AST (aspartate transaminase) in U/L
Yellow line: Bilirubin (total) in µmol/L
Gray line: Alkaline phosphatase in IU/L
Figure one: Trend of liver associated enzymes by presentation day
Orange line: ALT (alanine transaminase) in U/L
Blue line: AST (aspartate transaminase) in U/L
Yellow line: Bilirubin (total) in µmol/L
Gray line: Alkaline phosphatase in IU/L
Figure one: Trend of liver associated enzymes by presentation day
Disclosures:
Jessica Lilley indicated no relevant financial relationships.
Maunoo Lee indicated no relevant financial relationships.
Brett Sadowski indicated no relevant financial relationships.
Jessica L. Lilley, MD, Maunoo Lee, MD, Brett Sadowski, MD. P1014 - Severe, Protracted Cholestatic Liver Injury Due to Novel Fat Burner Albutarex V2 in an Active Duty Sailor, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
