Sunday Poster Session
Category: Liver
P1124 - Harm From SARMs: RAD-140, A Selective Androgen Receptor Modulator Leading to Acute Liver Injury
Sunday, October 22, 2023
3:30 PM - 7:00 PM PT
Location: Exhibit Hall
Has Audio
.jpg)
Alexander Pazevic, DO
Naval Medical Center
San Diego, California
Presenting Author(s)
Alex Pazevic, DO, Kevin Pak, MD, Angela Bachmann, MD, Brett Sadowski, MD
Naval Medical Center, San Diego, CA
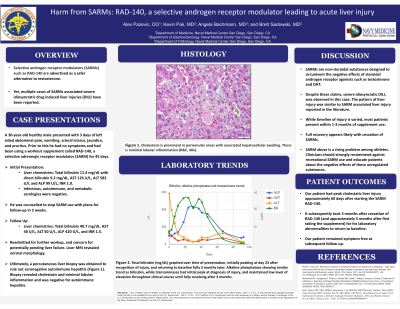
Introduction: RAD-140 (Testolone), a novel selective androgen receptor modulator (SARM) is currently undergoing trials for treatment of metastatic breast cancer and has found a niche market in the setting of exercise supplementation. SARMs are advertised as a safer alternative to testosterone without hepatotoxic side effects and are easily accessible via online marketplace. However, a growing body of evidence suggests these agents do indeed present risk for severe idiosyncratic drug induced liver injury (DILI). We present a case of severe cholestatic liver injury due to RAD-140 use in an otherwise healthy active duty servicemember.
Case Description/Methods: A 30-year-old male presented with 3 days of abdominal pain and pruritis. Prior to this he had no symptoms and had been using a workout supplement called RAD-140, a SARM for 45 days. His vitals were normal, and exam was notable for jaundice with left upper quadrant tenderness. CBC and BMP were normal. Liver enzymes revealed a total bilirubin 11.4 mg/dL with direct bili 9.2 mg/dL, AST 126 U/L, ALT 582 U/L and ALP 99 U/L. The INR was 1.0. Infectious, autoimmune, and metabolic serologies were negative. He was counseled to stop SARM use and had 2 week follow up, but unfortunately, his jaundice progressed. Follow up labs showed a bilirubin which had now risen to 40.7 mg/dL, ALP 420 U/L, ALT 50 U/L, and AST 40 U/L. INR rose to 1.5 without evidence of encephalopathy. Liver MRI revealed normal morphology. Ultimately, the liver was biopsied which showed extensive cholestasis and hepatocellular swelling and degeneration. There was no evidence of autoimmune hepatitis or fibrosis. Findings on liver biopsy in conjunction with his history were consistent with idiosyncratic liver injury secondary to SARM use. By 4 weeks, his symptoms and labs had begun to improve.
Discussion: SARMs are designed to circumvent the negative effects of steroidal androgen receptor agonists such as testosterone and DHT. However, recent case reports have shown quite profound cholestatic liver injuries due to RAD-140 alongside other SARMs. Our case’s clinical course was quite similar with previous reports, furthering the strength of this association, and disease pattern. Full recovery appears likely with cessation of medication. Despite the FDA warning against SARM use in body-building products, SARM abuse is a rising problem among athletes. Clinicians should strongly recommend against recreational SARM use and educate patients about the negative effects of these unregulated substances.
Disclosures:
Alex Pazevic, DO, Kevin Pak, MD, Angela Bachmann, MD, Brett Sadowski, MD. P1124 - Harm From SARMs: RAD-140, A Selective Androgen Receptor Modulator Leading to Acute Liver Injury, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
Naval Medical Center, San Diego, CA
Introduction: RAD-140 (Testolone), a novel selective androgen receptor modulator (SARM) is currently undergoing trials for treatment of metastatic breast cancer and has found a niche market in the setting of exercise supplementation. SARMs are advertised as a safer alternative to testosterone without hepatotoxic side effects and are easily accessible via online marketplace. However, a growing body of evidence suggests these agents do indeed present risk for severe idiosyncratic drug induced liver injury (DILI). We present a case of severe cholestatic liver injury due to RAD-140 use in an otherwise healthy active duty servicemember.
Case Description/Methods: A 30-year-old male presented with 3 days of abdominal pain and pruritis. Prior to this he had no symptoms and had been using a workout supplement called RAD-140, a SARM for 45 days. His vitals were normal, and exam was notable for jaundice with left upper quadrant tenderness. CBC and BMP were normal. Liver enzymes revealed a total bilirubin 11.4 mg/dL with direct bili 9.2 mg/dL, AST 126 U/L, ALT 582 U/L and ALP 99 U/L. The INR was 1.0. Infectious, autoimmune, and metabolic serologies were negative. He was counseled to stop SARM use and had 2 week follow up, but unfortunately, his jaundice progressed. Follow up labs showed a bilirubin which had now risen to 40.7 mg/dL, ALP 420 U/L, ALT 50 U/L, and AST 40 U/L. INR rose to 1.5 without evidence of encephalopathy. Liver MRI revealed normal morphology. Ultimately, the liver was biopsied which showed extensive cholestasis and hepatocellular swelling and degeneration. There was no evidence of autoimmune hepatitis or fibrosis. Findings on liver biopsy in conjunction with his history were consistent with idiosyncratic liver injury secondary to SARM use. By 4 weeks, his symptoms and labs had begun to improve.
Discussion: SARMs are designed to circumvent the negative effects of steroidal androgen receptor agonists such as testosterone and DHT. However, recent case reports have shown quite profound cholestatic liver injuries due to RAD-140 alongside other SARMs. Our case’s clinical course was quite similar with previous reports, furthering the strength of this association, and disease pattern. Full recovery appears likely with cessation of medication. Despite the FDA warning against SARM use in body-building products, SARM abuse is a rising problem among athletes. Clinicians should strongly recommend against recreational SARM use and educate patients about the negative effects of these unregulated substances.
Disclosures:
Alex Pazevic indicated no relevant financial relationships.
Kevin Pak indicated no relevant financial relationships.
Angela Bachmann indicated no relevant financial relationships.
Brett Sadowski indicated no relevant financial relationships.
Alex Pazevic, DO, Kevin Pak, MD, Angela Bachmann, MD, Brett Sadowski, MD. P1124 - Harm From SARMs: RAD-140, A Selective Androgen Receptor Modulator Leading to Acute Liver Injury, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
