Sunday Poster Session
Category: IBD
P0696 - Outcomes of Budesonide as a Treatment Option for Immune Checkpoint Inhibitor Related Colitis in Patients with Cancer

Antonio Pizuorno Machado, MD
University of Texas Health Science Center
Houston, TX
Presenting Author(s)
1University of Texas Health Science Center, Houston, TX; 2MD Anderson Cancer Center, Houston, TX; 3University of Texas MD Anderson Cancer Center, Houston, TX
Introduction: Budesonide is routinely employed in the management for inflammatory bowel conditions such as Crohn's disease and ulcerative colitis. Previous studies have shown vast similitudes in the spectrum of those conditions and colitis secondary to immune checkpoint inhibitors (ICI). Currently, the use of budesonide is limited to cases of less severe colitis although there is no available data on it’s role for long term prophylaxis and/or as a bridge from systemic corticosteroids. In this study we aimed to describe the characteristics and clinical profile of patients with ICI colitis that received budesonide.
Methods: This is a retrospective analysis that included adult cancer patients diagnosed with colitis secondary to ICI between 1/1/2015 and 11/31/2022 and received budesonide as treatment for primary colitis or other immune related adverse event (irAE). We report data on those that developed colitis up to 6 months after the last dose of ICI confirmed by laboratory and/or imaging report. We included patients’ demographic characteristics, oncologic profile, clinical course, endoscopic features as well as treatment and outcomes.
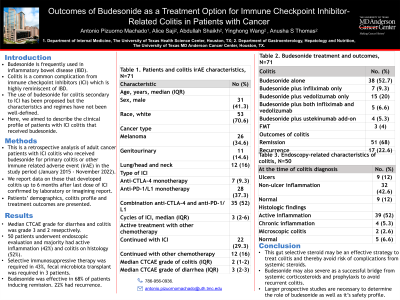
Results: Our sample (n=72) comprised primarily of Caucasian (70.6%) females (58.7%) where the most common malignancy was melanoma (34.6%). The majority of these patients received combination therapy of anti-PD1/L1 and CTLA-4 (52%). Budesonide was more commonly used primarily to treat colitis (52%), followed by bridging therapy from systemic corticosteroids (30.6%). The median CTCAE grade of diarrhea was 3 and colitis was 2 with only few patients (8.3%) with colitis over grade 3. On endoscopic evaluation, a majority of patients had non ulcer inflammation (42.6%) and active colitis on histology (52%). Less than half of the patients required additional therapies (43%). 68% of patients achieved remission with 22.6% experiencing recurrence. After budesonide, 29.3% had ICI resumed and 16% continued with other forms of cancer treatment.
Discussion: Budesonide may be an effective strategy to both treat and prevent colitis especially to substitute systemic corticosteroids; the remission rates observed with budesonide alone are comparable. Further we observe that budesonide may also serve as a successful bridge from systemic corticosteroids and may be considered for prophylaxis to avoid recurrent colitis. Larger prospective studies are necessary to determine the role of budesonide as well as its safety profile.
Table 1. Characteristics of gastrointestinal irAE in patients with colorectal cancer and Endoscopy-related characteristics for patients diagnosed with colitis, N=72 | |
Colitis, N=72 | No. (%) |
Symptoms |
|
Diarrhea | 70 (93.3) |
Abdominal pain | 65 (86.6) |
No symptoms | 2 (2.6) |
Median fecal calprotectin before treatment, (IQR) | 671 (106.9-1000) |
Median CTCAE grade of colitis (IQR) | 2 (1-2) |
Median CTCAE grade of diarrhea (IQR) | 3 (2-3) |
Hospitalization required | 35 (46.6) |
At the time of colitis diagnosis, N=50 | No. (%) |
Endoscopic findings (only those that underwent endoscopy), N=50 |
|
Ulcers | 9 (12) |
Non-ulcer inflammation | 32 (42.6) |
Normal | 9 (12) |
Histologic findings |
|
Active inflammation | 39 (52) |
Chronic inflammation | 4 (5.3) |
Microscopic colitis | 2 (2.6) |
Normal | 5 (6.6) |
Treatment of IMC |
|
Budesonide alone | 38 (52.7) |
Budesonide plus infliximab only | 7 (9.3) |
Budesonide plus vedolizumab only | 15 (20) |
Budesonide plus both infliximab and vedolizumab | 5 (6.6) |
Budesonide plus ustekinumab add-on | 4 (5.3) |
FMT | 3 (4) |
Complications of IMC | 2 (2.6) |
Characteristics of budesonide use, N=72 | No. (%) |
Type of use in regards of budesonide |
|
Primarily to treat IMC | 39 (52) |
Bridged from systemic steroid | 23 (30.6) |
Prophylactic use | 4 (5.3) |
Other use | 2 (2.7) |
Budesonide use |
|
Median time of budesonide use, days | 42.5 (28-107) |
Outcomes of colitis |
|
Remission | 51 (68) |
Recurrence | 17 (22.6) |
Abbreviations: CTCAE v5, Common Terminology Criteria for Adverse Events version 5; ICI, immune checkpoint inhibitor; IMC, immune-mediated colitis; IQR, interquartile range; TNF, tumor necrosis factor; FMT, fecal microbiota transplantation; irAE, immune related adverse event | |
Disclosures:
Antonio Pizuorno Machado, MD1, Alice Saji, DNP, MSN, APN2, Abdullah S. Shaikh, MD3, Yinghong Wang, MD3, Anusha Thomas, MD3. P0696 - Outcomes of Budesonide as a Treatment Option for Immune Checkpoint Inhibitor Related Colitis in Patients with Cancer, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
