Monday Poster Session
Category: Esophagus
P1838 - Patient Preferences for Medical Treatment of Eosinophilic Esophagitis
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
Has Audio

David Leiman, MD, MSHP
Duke University Medical Center
Durham, NC
Presenting Author(s)
David Leiman, MD, MSHP1, F Reed. Johnson, PhD2, Mary Jo Strobel, 3, Jessie Sutphin, MA2
1Duke University Medical Center, Durham, NC; 2Duke Clinical Research Institute, Durham, NC; 3AFPED, Atlanta, GA
Introduction: Eosinophilic esophagitis (EoE) is chronic allergic inflammatory condition associated with esophageal dysfunction. Several medical treatment options exist, including proton pump inhibitors (PPIs), swallowed topical steroids (STS), and biologic therapy, which vary by efficacy, side effects, and other attributes. Patients must balance these tradeoffs when selecting treatments and knowledge of these preferences is limited. We aimed to quantify patient preferences for medical management of EoE.
Methods: Patients ≥18 years with a confirmed or self-reported diagnosis of EoE, identified through disease-specific diagnostic codes or by membership in a patient advocacy organization, completed an online survey eliciting EoE-specific treatment preferences and clinicodemographic information. A discrete choice experiment (DCE) was constructed to quantify the importance of four treatment attributes: time without symptoms (10, 5 or 2 months), chance of no inflammation defined by histologic remission with < 15 eosinophils/hpf at 12 months (90, 60, or 40%), regulatory approval for EoE, and treatment type (PPI, STS, or biologic therapy). Respondents completed 9 experimentally designed DCE questions. Data were analyzed with a random-parameters logit model to elicit relative preference weights (PW) for each treatment attribute.
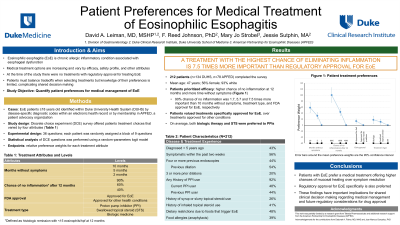
Results: 212 respondents completed the survey (56% female; 93% white) with a mean age of 47 years and 56% reported EoE symptoms within the prior two weeks (Table 1). Patients preferred treatments offering a higher chance of no inflammation at 12 months (90% PW 100; 60% PW 43.8) and more time without symptoms (10 months PW 58.2; 5 months PW 31.2) (Figure 1). Patients also valued a treatment with regulatory approval specifically for EoE (PW 13.3) over one approved for other conditions. There was no significant difference in preferences for biologic therapy (PW 17.6) or STS (PW 11.0), though both were preferred to PPIs. Overall, a 90% chance of no inflammation was 1.7, 5.7 and 7.5 times more important than 10 months without symptoms, treatment type, and having FDA approval for EoE, respectively.
Discussion: Patients with EoE have increasing options for medical therapy and prefer a treatment offering higher chances of mucosal healing over symptom resolution. Regulatory approval for EoE is also preferred. These findings have important implications for shared clinical decision making regarding medical management and future regulatory considerations for drug approval.

Disclosures:
David Leiman, MD, MSHP1, F Reed. Johnson, PhD2, Mary Jo Strobel, 3, Jessie Sutphin, MA2. P1838 - Patient Preferences for Medical Treatment of Eosinophilic Esophagitis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Duke University Medical Center, Durham, NC; 2Duke Clinical Research Institute, Durham, NC; 3AFPED, Atlanta, GA
Introduction: Eosinophilic esophagitis (EoE) is chronic allergic inflammatory condition associated with esophageal dysfunction. Several medical treatment options exist, including proton pump inhibitors (PPIs), swallowed topical steroids (STS), and biologic therapy, which vary by efficacy, side effects, and other attributes. Patients must balance these tradeoffs when selecting treatments and knowledge of these preferences is limited. We aimed to quantify patient preferences for medical management of EoE.
Methods: Patients ≥18 years with a confirmed or self-reported diagnosis of EoE, identified through disease-specific diagnostic codes or by membership in a patient advocacy organization, completed an online survey eliciting EoE-specific treatment preferences and clinicodemographic information. A discrete choice experiment (DCE) was constructed to quantify the importance of four treatment attributes: time without symptoms (10, 5 or 2 months), chance of no inflammation defined by histologic remission with < 15 eosinophils/hpf at 12 months (90, 60, or 40%), regulatory approval for EoE, and treatment type (PPI, STS, or biologic therapy). Respondents completed 9 experimentally designed DCE questions. Data were analyzed with a random-parameters logit model to elicit relative preference weights (PW) for each treatment attribute.
Results: 212 respondents completed the survey (56% female; 93% white) with a mean age of 47 years and 56% reported EoE symptoms within the prior two weeks (Table 1). Patients preferred treatments offering a higher chance of no inflammation at 12 months (90% PW 100; 60% PW 43.8) and more time without symptoms (10 months PW 58.2; 5 months PW 31.2) (Figure 1). Patients also valued a treatment with regulatory approval specifically for EoE (PW 13.3) over one approved for other conditions. There was no significant difference in preferences for biologic therapy (PW 17.6) or STS (PW 11.0), though both were preferred to PPIs. Overall, a 90% chance of no inflammation was 1.7, 5.7 and 7.5 times more important than 10 months without symptoms, treatment type, and having FDA approval for EoE, respectively.
Discussion: Patients with EoE have increasing options for medical therapy and prefer a treatment offering higher chances of mucosal healing over symptom resolution. Regulatory approval for EoE is also preferred. These findings have important implications for shared clinical decision making regarding medical management and future regulatory considerations for drug approval.

Figure: Patient preference weights for medical therapy of eosinophilic esophagitis (EoE) with 95% confidence intervals.
Disclosures:
David Leiman: Astra Zeneca – Consultant. Bristol Myers Squibb – Stock-publicly held company(excluding mutual/index funds). Medtronic – Consultant. Novo Nordisk – Consultant. Sanofi – Advisor or Review Panel Member. Takeda – Grant/Research Support.
F Johnson: Takeda – Grant/Research Support.
Mary Jo Strobel indicated no relevant financial relationships.
Jessie Sutphin: Takeda – Grant/Research Support.
David Leiman, MD, MSHP1, F Reed. Johnson, PhD2, Mary Jo Strobel, 3, Jessie Sutphin, MA2. P1838 - Patient Preferences for Medical Treatment of Eosinophilic Esophagitis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
