Monday Poster Session
Category: IBD
P2139 - Exploring the Influence of COVID-19 Treatment on Real-World Outcomes in IBD Patients: A Comprehensive Analysis
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
Has Audio
- LS
Laura Sahyoun, MD
Yale University
New Haven, CT
Presenting Author(s)
Laura Sahyoun, MD1, Chandler McMillan, BA2, Jonathan Fetene, MD3, Petr Protiva, MD, MPH4, Badr Al-Bawardy, MD5, Jill Gaidos, MD, FACG1, Deborah D. Proctor, MD6
1Yale University, New Haven, CT; 2Yale University School of Medicine, New Haven, CT; 3Yale New Haven Hospital, New Haven, CT; 4Yale University School of Medicine and VA Connecticut Healthcare System, New Haven, CT; 5King Faisal Specialist Hospital and Research Center, Riyadh, Ar Riyad, Saudi Arabia; 6Yale University, Durham, CT
Introduction: There are multiple safe and effective antiviral agents and monoclonal antibodies for COVID-19 treatment. The impact of these treatments in patients with inflammatory bowel disease (IBD) remains uncertain. Our objective was to assess the effects of these therapies on outcomes related to both conditions.
Methods: This single-center retrospective study involved adult IBD patients who contracted COVID-19 between 12/2020 to 11/2022. The study analyzed data on current IBD treatments, interventions for COVID-19 infection, and mortality. Advanced therapies consisted of biologics and small molecules. Patients were stratified by COVID-19 treatment (antivirals and/or intravenous (IV) antibodies) vs no therapy. The primary outcome aimed to determine the impact of these treatments on the development of severe COVID-19 infection, defined by need for supplemental oxygen, corticosteroids and/or antibiotics, or hospitalization. Secondary outcomes encompassed rates of withholding IBD therapy and rates of post-COVID IBD flare.
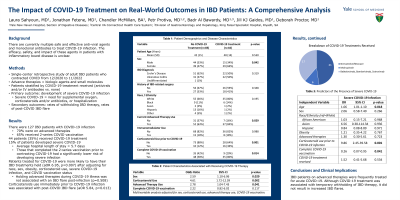
Results: Out of the 127 subjects who met study criteria, 35% received COVID-19 treatment. Overall, 70% of subjects were on advanced therapies, 54% were female, and 58% had CD. Those treated for COVID-19 were more likely to be on corticosteroids [odds ratio (OR) 4.17, 95% confidence interval (CI) 1.69-10.28] or advanced IBD therapy (OR 3.15, 95% CI 1.25-7.92). After adjusting for age, race, sex, steroid use, obesity, COVID-19 vaccination status, and severe COVID-19 infection, those treated for COVID-19 were more likely to have IBD therapy held (OR 6.95, 95% CI 1.72-28.15) [Table 1]. Advanced therapies were held for an average of 21 days, consistent with institutional recommendations. There was no significant difference in the occurrence of a post-COVID IBD flare or severe COVID-19 infection between those who were treated and those who were not. Among the 19 subjects with severe COVID-19 infection, 6 were on biologics, 5 on no IBD therapy, 5 on combination therapy with a biologic and immunomodulator, 2 on tofacitinib, and 1 on anti-TNF and tofacitinib. There were no COVID-related deaths.
Discussion: In our study, IBD patients on immunosuppressant medications were frequently treated with anti-viral or IV antibody therapies for acute COVID-19. Importantly, we found inverse association between the use of these therapies and the development of severe COVID-19. Although COVID-19 treatment was associated with temporary withholding of IBD therapy, it did not result in increased in IBD flares.
Disclosures:
Laura Sahyoun, MD1, Chandler McMillan, BA2, Jonathan Fetene, MD3, Petr Protiva, MD, MPH4, Badr Al-Bawardy, MD5, Jill Gaidos, MD, FACG1, Deborah D. Proctor, MD6. P2139 - Exploring the Influence of COVID-19 Treatment on Real-World Outcomes in IBD Patients: A Comprehensive Analysis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Yale University, New Haven, CT; 2Yale University School of Medicine, New Haven, CT; 3Yale New Haven Hospital, New Haven, CT; 4Yale University School of Medicine and VA Connecticut Healthcare System, New Haven, CT; 5King Faisal Specialist Hospital and Research Center, Riyadh, Ar Riyad, Saudi Arabia; 6Yale University, Durham, CT
Introduction: There are multiple safe and effective antiviral agents and monoclonal antibodies for COVID-19 treatment. The impact of these treatments in patients with inflammatory bowel disease (IBD) remains uncertain. Our objective was to assess the effects of these therapies on outcomes related to both conditions.
Methods: This single-center retrospective study involved adult IBD patients who contracted COVID-19 between 12/2020 to 11/2022. The study analyzed data on current IBD treatments, interventions for COVID-19 infection, and mortality. Advanced therapies consisted of biologics and small molecules. Patients were stratified by COVID-19 treatment (antivirals and/or intravenous (IV) antibodies) vs no therapy. The primary outcome aimed to determine the impact of these treatments on the development of severe COVID-19 infection, defined by need for supplemental oxygen, corticosteroids and/or antibiotics, or hospitalization. Secondary outcomes encompassed rates of withholding IBD therapy and rates of post-COVID IBD flare.
Results: Out of the 127 subjects who met study criteria, 35% received COVID-19 treatment. Overall, 70% of subjects were on advanced therapies, 54% were female, and 58% had CD. Those treated for COVID-19 were more likely to be on corticosteroids [odds ratio (OR) 4.17, 95% confidence interval (CI) 1.69-10.28] or advanced IBD therapy (OR 3.15, 95% CI 1.25-7.92). After adjusting for age, race, sex, steroid use, obesity, COVID-19 vaccination status, and severe COVID-19 infection, those treated for COVID-19 were more likely to have IBD therapy held (OR 6.95, 95% CI 1.72-28.15) [Table 1]. Advanced therapies were held for an average of 21 days, consistent with institutional recommendations. There was no significant difference in the occurrence of a post-COVID IBD flare or severe COVID-19 infection between those who were treated and those who were not. Among the 19 subjects with severe COVID-19 infection, 6 were on biologics, 5 on no IBD therapy, 5 on combination therapy with a biologic and immunomodulator, 2 on tofacitinib, and 1 on anti-TNF and tofacitinib. There were no COVID-related deaths.
Discussion: In our study, IBD patients on immunosuppressant medications were frequently treated with anti-viral or IV antibody therapies for acute COVID-19. Importantly, we found inverse association between the use of these therapies and the development of severe COVID-19. Although COVID-19 treatment was associated with temporary withholding of IBD therapy, it did not result in increased in IBD flares.
Disclosures:
Laura Sahyoun indicated no relevant financial relationships.
Chandler McMillan indicated no relevant financial relationships.
Jonathan Fetene indicated no relevant financial relationships.
Petr Protiva indicated no relevant financial relationships.
Badr Al-Bawardy: AbbVie – Speakers Bureau. Bristol Myers Squibb – Advisor or Review Panel Member, Speakers Bureau. Pfizer – Advisor or Review Panel Member. Takeda – Speakers Bureau.
Jill Gaidos: Abbvie – Advisor or Review Panel Member, Grant/Research Support. Bristol Myers Squibb – Grant/Research Support. Pfizer – Consultant, Grant/Research Support.
Deborah Proctor: Abbvie – Consultant, Grant/Research Support. BMS – Grant/Research Support. Janssen – Grant/Research Support. Pfizer – Grant/Research Support.
Laura Sahyoun, MD1, Chandler McMillan, BA2, Jonathan Fetene, MD3, Petr Protiva, MD, MPH4, Badr Al-Bawardy, MD5, Jill Gaidos, MD, FACG1, Deborah D. Proctor, MD6. P2139 - Exploring the Influence of COVID-19 Treatment on Real-World Outcomes in IBD Patients: A Comprehensive Analysis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
