Monday Poster Session
Category: IBD
P2167 - Characterization of a First-in-Class Oral Therapy Selectively Targeting the IL-23 Pathway
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
Has Audio

Anne Fourie, MSc, PhD
Janssen Research & Development, LLC
La Jolla, CA
Presenting Author(s)
Anne Fourie, MSc, PhD1, Xiaoli Cheng, PhD2, Leon Chang, BSc1, Carrie Greving, BSc, PhD1, Aaron Patrick, BS, PhD3, Beverly Knight, PhD4, David Polidori, BS, MS, PhD1, Raymond Patch, PhD5, Ashok Bhandari, PhD6, David Liu, PhD6, Keith Huie, MSc7, Shu Li, MSc6, Michael Rodriguez, BSc8, Arun Kannan, MS, PhD9, Jonathan Sherlock, MB, BChir, PhD8, Nishit Modi, MBA, PhD6
1Janssen Research & Development, LLC, La Jolla, CA; 2Anwita Biosciences, San Carlos, CA; 3Janssen Research and Development LLC, Spring House, PA; 4Janseen Research and Development LLC, La Jolla, CA; 5Janssen Research & Development, LLC, Spring House, NJ; 6Protagonist Therapeutics Inc., Newark, CA; 7Cytokinetics, South San Francisco, CA; 8Janssen Research & Development, LLC, Spring House, PA; 9Janseen Research and Development LLC, Spring House, PA
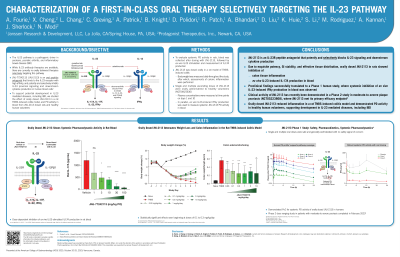
Introduction: The IL-23 pathway is a pathogenic driver in psoriasis, psoriatic arthritis, and inflammatory bowel disease. While IL-23 antibody therapies are available, there are currently no orally delivered therapies selectively targeting this pathway.
JNJ-77242113 (JNJ-2113) is an oral peptide antagonist that binds to the IL-23 receptor with high affinity, and potently and selectively inhibits IL-23 proximal signaling and downstream cytokine production in human blood cells.*
To support potential development in IL-23 mediated diseases, we studied the effect of orally dosed JNJ-2113 in a rat TNBS-induced colitis model, and pharmacodynamic (PD) activity in blood from JNJ-2113 dosed rats and healthy human volunteers.
Methods: To evaluate systemic PD activity in rats, blood was collected after dosing with JNJ-2113, followed by ex vivo IL-23-stimulation and measurement of IL-17A production.
JNJ-2113 was dosed orally in a rat model of TNBS-induced colitis. Body weight was measured daily throughout the study, after which assessments of colonic inflammation were performed.
Single and multiple ascending doses of JNJ-2113 were orally administered to healthy volunteers (HV) (NCT04621630). Plasma concentrations were measured at time points on days 1 and 10, and in parallel ex vivo IL-23-induced IFNγ production was used to measure systemic JNJ-2113 PD activity in blood.
Results: After oral dosing of JNJ-2113 in rats, dose-dependent inhibition of ex vivo IL-23-stimulated IL-17A production in blood was observed.
In rat TNBS-induced colitis, oral dosing of JNJ-2113 significantly attenuated weight loss and colon inflammation (Figure 1, A and B respectively) at doses as low as 0.1 mg/kg/day.
In HV, single and multiple oral doses of JNJ-2113 were well tolerated with no safety signal of concern. Oral dosing of JNJ-2113 resulted in increasing exposures across the dose range and inhibition of ex vivo IL-23 stimulated IFN production in whole blood. Thus, JNJ-2113 demonstrated robust systemic PD activity and IL-23 pathway engagement in humans.
Discussion: : These data support the potential for JNJ-2113 as a first-in-class oral therapy targeting IL-23-mediated diseases. Of note, clinical activity of JNJ-2113 has recently been demonstrated in a Phase 2 study in moderate-to-severe plaque psoriasis patients (NCT05223868) where JNJ-2113 met its primary efficacy endpoint 2.
1Fourie et al. JID 143(5) S190, May 2023
2 https://feeds.issuerdirect.com/news-release.html?newsid=6598557738756234

Disclosures:
Anne Fourie, MSc, PhD1, Xiaoli Cheng, PhD2, Leon Chang, BSc1, Carrie Greving, BSc, PhD1, Aaron Patrick, BS, PhD3, Beverly Knight, PhD4, David Polidori, BS, MS, PhD1, Raymond Patch, PhD5, Ashok Bhandari, PhD6, David Liu, PhD6, Keith Huie, MSc7, Shu Li, MSc6, Michael Rodriguez, BSc8, Arun Kannan, MS, PhD9, Jonathan Sherlock, MB, BChir, PhD8, Nishit Modi, MBA, PhD6. P2167 - Characterization of a First-in-Class Oral Therapy Selectively Targeting the IL-23 Pathway, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Janssen Research & Development, LLC, La Jolla, CA; 2Anwita Biosciences, San Carlos, CA; 3Janssen Research and Development LLC, Spring House, PA; 4Janseen Research and Development LLC, La Jolla, CA; 5Janssen Research & Development, LLC, Spring House, NJ; 6Protagonist Therapeutics Inc., Newark, CA; 7Cytokinetics, South San Francisco, CA; 8Janssen Research & Development, LLC, Spring House, PA; 9Janseen Research and Development LLC, Spring House, PA
Introduction: The IL-23 pathway is a pathogenic driver in psoriasis, psoriatic arthritis, and inflammatory bowel disease. While IL-23 antibody therapies are available, there are currently no orally delivered therapies selectively targeting this pathway.
JNJ-77242113 (JNJ-2113) is an oral peptide antagonist that binds to the IL-23 receptor with high affinity, and potently and selectively inhibits IL-23 proximal signaling and downstream cytokine production in human blood cells.*
To support potential development in IL-23 mediated diseases, we studied the effect of orally dosed JNJ-2113 in a rat TNBS-induced colitis model, and pharmacodynamic (PD) activity in blood from JNJ-2113 dosed rats and healthy human volunteers.
Methods: To evaluate systemic PD activity in rats, blood was collected after dosing with JNJ-2113, followed by ex vivo IL-23-stimulation and measurement of IL-17A production.
JNJ-2113 was dosed orally in a rat model of TNBS-induced colitis. Body weight was measured daily throughout the study, after which assessments of colonic inflammation were performed.
Single and multiple ascending doses of JNJ-2113 were orally administered to healthy volunteers (HV) (NCT04621630). Plasma concentrations were measured at time points on days 1 and 10, and in parallel ex vivo IL-23-induced IFNγ production was used to measure systemic JNJ-2113 PD activity in blood.
Results: After oral dosing of JNJ-2113 in rats, dose-dependent inhibition of ex vivo IL-23-stimulated IL-17A production in blood was observed.
In rat TNBS-induced colitis, oral dosing of JNJ-2113 significantly attenuated weight loss and colon inflammation (Figure 1, A and B respectively) at doses as low as 0.1 mg/kg/day.
In HV, single and multiple oral doses of JNJ-2113 were well tolerated with no safety signal of concern. Oral dosing of JNJ-2113 resulted in increasing exposures across the dose range and inhibition of ex vivo IL-23 stimulated IFN production in whole blood. Thus, JNJ-2113 demonstrated robust systemic PD activity and IL-23 pathway engagement in humans.
Discussion: : These data support the potential for JNJ-2113 as a first-in-class oral therapy targeting IL-23-mediated diseases. Of note, clinical activity of JNJ-2113 has recently been demonstrated in a Phase 2 study in moderate-to-severe plaque psoriasis patients (NCT05223868) where JNJ-2113 met its primary efficacy endpoint 2.
1Fourie et al. JID 143(5) S190, May 2023
2 https://feeds.issuerdirect.com/news-release.html?newsid=6598557738756234

Figure: Figure 1. Rat TNBS-induced Colitis Model Results: A) body weight changes; B) colon edema and shortening; ns=not significant, **p <0.01, ****p <0.0001
Disclosures:
Anne Fourie: Janssen Research and Development – Employee, May own stock/stock options in Johnson & Johnson, of which Janssen is a subsidiary.
Xiaoli Cheng indicated no relevant financial relationships.
Leon Chang: Janssen Research and Development – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Carrie Greving: Janssen Research and Development – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Aaron Patrick: Janssen Research and Development – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Beverly Knight: Janssen Research and Development – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
David Polidori: Janssen – Employee, Stock-publicly held company(excluding mutual/index funds).
Raymond Patch: Janssen Research and Develpment – Employee, Former employees of Janssen Research & Development, LLC; employees may own stock/stock options in Johnson & Johnson, of which Janssen is a.
Ashok Bhandari indicated no relevant financial relationships.
David Liu indicated no relevant financial relationships.
Keith Huie: Cytokinetics – Employee. Protagonist Therapeutics – Employee.
Shu Li indicated no relevant financial relationships.
Michael Rodriguez: Janssen Research & Development., LLC – Employee, Intellectual Property/Patents, Stock Options.
Arun Kannan: Janssen Research adn Development – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Jonathan Sherlock: Janssen – Employee.
Nishit Modi indicated no relevant financial relationships.
Anne Fourie, MSc, PhD1, Xiaoli Cheng, PhD2, Leon Chang, BSc1, Carrie Greving, BSc, PhD1, Aaron Patrick, BS, PhD3, Beverly Knight, PhD4, David Polidori, BS, MS, PhD1, Raymond Patch, PhD5, Ashok Bhandari, PhD6, David Liu, PhD6, Keith Huie, MSc7, Shu Li, MSc6, Michael Rodriguez, BSc8, Arun Kannan, MS, PhD9, Jonathan Sherlock, MB, BChir, PhD8, Nishit Modi, MBA, PhD6. P2167 - Characterization of a First-in-Class Oral Therapy Selectively Targeting the IL-23 Pathway, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
