Monday Poster Session
Category: IBD
P2178 - Onset of the COVID-19 Pandemic Did Not Negatively Impact Adherence to Vedolizumab Among IBD Patients
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
Has Audio
- CL
Christopher Lindholm, MD
University of Wisconsin-Madison
Madison, WI
Presenting Author(s)
Lindholm Christopher, MD1, Mary Nemer, MD1, Lucas Fass, MD2, Fauzia Osman, MPH2, Freddy Caldera, DO, MS3, Sumona Saha, MD4
1University of Wisconsin-Madison, Madison, WI; 2University of Wisconsin Hospital and Clinics, Madison, WI; 3University of Wisconsin School of Medicine and Public Health, Madison, WI; 4University of Wisconsin, Milwaukee, WI
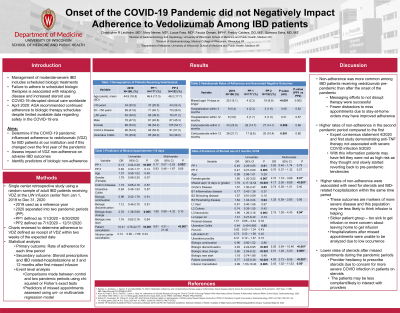
Introduction: The onset of the COVID-19 pandemic caused numerous disruptions in patient care; however, per expert opinion and early guidance from national GI societies IBD patients were encouraged to continue biologic therapy. The goal of this study was to assess adherence to vedolizumab among IBD patients during the pandemic, associate adherence rates with need for steroids and IBD-related hospitalization and identify factors associated with non-adherence.
Methods: We performed a single center retrospective cohort study at the University of Wisconsin-Madison. IBD patients ordered to receive vedolizumab from 1/1/19 to 12/31/20 were included. Two pandemic periods (PP) were assessed: 1/1/20-6/30/20 (PP1) and 7/1/20- 12/31/20 (PP2) to evaluate the effect of different phases of the pandemic on infusion adherence. Adherence to therapy was defined as receiving vedolizumab within 14 days of the scheduled date. The primary outcome was the rate of adherence during each time period. Secondary outcomes included corticosteroid prescriptions and IBD-related hospitalizations at 3- and 12-months after the first missed infusion appointment for each specified time period. Comparisons were made between 2019 (reference period) and the two PPs using chi-squared or Fisher’s exact tests. Comparisons between groups and continuous data were done using ANOVA or non-parametric Wilcoxon sign rank tests. Predictors of non-adherent events and secondary outcomes were assessed using univariate and multivariate logistic regression models.
Results: 166 patients received vedolizumab in 2019, 177 patients in PP1 and 192 patients in PP2. Rates of non-adherence were higher in 2019 (pre-pandemic period) compared to PP1 and PP2. There were fewer corticosteroid prescriptions administered at 3 and 12 months after a non-adherent event during the pandemic compared to 2019. There was no difference in IBD-related hospitalizations (Table 1). Non-adherence was positively associated with a patient-related reason for missing infusion appointment (OR=10.41, 95% CI [4.76-22.77], p< 0.001). Negative predictors of non-adherence included infusions in PP1 (OR=0.13, 95% CI [0.04-0.38], p< 0.001).
Discussion: Higher rates of non-adherence to vedolizumab among IBD patients were associated with worsened outcomes including corticosteroid use and IBD-related hospitalizations. Non-adherence was more common in the pre-pandemic period than after the onset of the pandemic suggesting messaging to not disrupt vedolizumab therapy during the pandemic was successful.
Disclosures:
Lindholm Christopher, MD1, Mary Nemer, MD1, Lucas Fass, MD2, Fauzia Osman, MPH2, Freddy Caldera, DO, MS3, Sumona Saha, MD4. P2178 - Onset of the COVID-19 Pandemic Did Not Negatively Impact Adherence to Vedolizumab Among IBD Patients, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1University of Wisconsin-Madison, Madison, WI; 2University of Wisconsin Hospital and Clinics, Madison, WI; 3University of Wisconsin School of Medicine and Public Health, Madison, WI; 4University of Wisconsin, Milwaukee, WI
Introduction: The onset of the COVID-19 pandemic caused numerous disruptions in patient care; however, per expert opinion and early guidance from national GI societies IBD patients were encouraged to continue biologic therapy. The goal of this study was to assess adherence to vedolizumab among IBD patients during the pandemic, associate adherence rates with need for steroids and IBD-related hospitalization and identify factors associated with non-adherence.
Methods: We performed a single center retrospective cohort study at the University of Wisconsin-Madison. IBD patients ordered to receive vedolizumab from 1/1/19 to 12/31/20 were included. Two pandemic periods (PP) were assessed: 1/1/20-6/30/20 (PP1) and 7/1/20- 12/31/20 (PP2) to evaluate the effect of different phases of the pandemic on infusion adherence. Adherence to therapy was defined as receiving vedolizumab within 14 days of the scheduled date. The primary outcome was the rate of adherence during each time period. Secondary outcomes included corticosteroid prescriptions and IBD-related hospitalizations at 3- and 12-months after the first missed infusion appointment for each specified time period. Comparisons were made between 2019 (reference period) and the two PPs using chi-squared or Fisher’s exact tests. Comparisons between groups and continuous data were done using ANOVA or non-parametric Wilcoxon sign rank tests. Predictors of non-adherent events and secondary outcomes were assessed using univariate and multivariate logistic regression models.
Results: 166 patients received vedolizumab in 2019, 177 patients in PP1 and 192 patients in PP2. Rates of non-adherence were higher in 2019 (pre-pandemic period) compared to PP1 and PP2. There were fewer corticosteroid prescriptions administered at 3 and 12 months after a non-adherent event during the pandemic compared to 2019. There was no difference in IBD-related hospitalizations (Table 1). Non-adherence was positively associated with a patient-related reason for missing infusion appointment (OR=10.41, 95% CI [4.76-22.77], p< 0.001). Negative predictors of non-adherence included infusions in PP1 (OR=0.13, 95% CI [0.04-0.38], p< 0.001).
Discussion: Higher rates of non-adherence to vedolizumab among IBD patients were associated with worsened outcomes including corticosteroid use and IBD-related hospitalizations. Non-adherence was more common in the pre-pandemic period than after the onset of the pandemic suggesting messaging to not disrupt vedolizumab therapy during the pandemic was successful.
Disclosures:
Lindholm Christopher indicated no relevant financial relationships.
Mary Nemer indicated no relevant financial relationships.
Lucas Fass indicated no relevant financial relationships.
Fauzia Osman indicated no relevant financial relationships.
Freddy Caldera: Arena – Consultant. Celgene – Consultant. GlaxoSmithKline – Consultant. Janssen – Grant/Research Support. Novavax – Grant/Research Support. Takeda – Consultant, Grant/Research Support.
Sumona Saha indicated no relevant financial relationships.
Lindholm Christopher, MD1, Mary Nemer, MD1, Lucas Fass, MD2, Fauzia Osman, MPH2, Freddy Caldera, DO, MS3, Sumona Saha, MD4. P2178 - Onset of the COVID-19 Pandemic Did Not Negatively Impact Adherence to Vedolizumab Among IBD Patients, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
