Monday Poster Session
Category: IBD
P2256 - Epstein-Barr Virus Associated Ulcers in a Patient with Ulcerative Colitis Taking Vedolizumab: A Case Report
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
Has Audio

Christopher Fernandes, BSc
Anne Burnett Marion School of Medicine at TCU
Fort Worth, TX
Presenting Author(s)
Christopher Fernandes, BSc1, Christine Catinis, MD, MS2, Precious Anyanwu, PharmD, PhD3, Timothy E.. Ritter, MD4
1Anne Burnett Marion School of Medicine at TCU, Fort Worth, TX; 2University of Texas Health Science Center at Houston, Houston, TX; 3Healix, LLC, Houston, TX; 4GI Alliance, Southlake, TX
Introduction: Wiskott-Aldrich Syndrome (WAS) is an X-linked recessive disorder characterized by immunodeficiency, eczema and microthrombocytopenia. The syndrome is caused by compromised function of the WASp protein resulting in a decreased ability of cells to polymerize and reorganize actin, which is crucial for various cellular processes such as cellular division, immune synapse formation, phagocytosis, antigen presentation, and immune surveillance and regulation.
The immune dysfunction in WAS can lead to the development of other autoimmune diseases and non-Hodgkin lymphoma. Additionally, individuals with WAS have an elevated risk of developing chronic inflammatory bowel disease. Here, we discuss a patient with WAS and ulcerative colitis (UC) receiving vedolizumab (VDZ) who developed an Epstein Barr virus (EBV) associated mucocutaneous rectal ulcer.
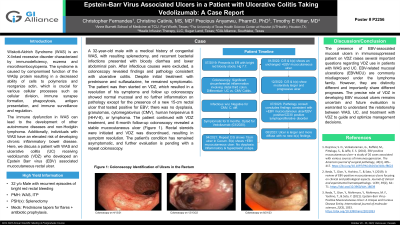
Case Description/Methods: A 32-year-old male with a medical history of congenital WAS, with resulting splenectomy, and recurrent bacterial infections presented with bloody diarrhea and lower abdominal pain. After infectious causes were excluded, a colonoscopy revealed findings and pathology consistent with ulcerative colitis. Despite initial treatment with prednisone and sulfasalazine, he remained symptomatic. The patient was then started on VDZ, which resulted in a resolution of his symptoms and follow up colonoscopy showed normal mucosal and no further inflammation on pathology except for the presence of a new 15-cm rectal ulcer that tested positive for EBV; there was no dysplasia, granulomas, cytomegalovirus (CMV), human herpesvirus 8 (HHV-8), or lymphoma. The patient continued with VDZ treatment, and 6-month follow-up colonoscopy revealed a stable mucocutaneous ulcer (Figure 1). Rectal steroids were initiated and VDZ was discontinued, resulting in symptom resolution. The patient's condition has remained asymptomatic, and further evaluation is pending with a repeat colonoscopy.
Discussion: The presence of EBV-associated mucosal ulcers in immunosuppressed patient on VDZ raises several important questions regarding VDZ use in patients with WAS and UC. EBV-related mucosal ulcerations (EBVMCU) are commonly misdiagnosed under the lymphoma family. However, they are distinctly different and importantly share different prognoses. The precise role of VDZ in developing EBVMCU remains uncertain and future evaluation is warranted to understand the relationship between WAS, UC, and treatment with VDZ to guide and optimize management decisions.

Disclosures:
Christopher Fernandes, BSc1, Christine Catinis, MD, MS2, Precious Anyanwu, PharmD, PhD3, Timothy E.. Ritter, MD4. P2256 - Epstein-Barr Virus Associated Ulcers in a Patient with Ulcerative Colitis Taking Vedolizumab: A Case Report, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Anne Burnett Marion School of Medicine at TCU, Fort Worth, TX; 2University of Texas Health Science Center at Houston, Houston, TX; 3Healix, LLC, Houston, TX; 4GI Alliance, Southlake, TX
Introduction: Wiskott-Aldrich Syndrome (WAS) is an X-linked recessive disorder characterized by immunodeficiency, eczema and microthrombocytopenia. The syndrome is caused by compromised function of the WASp protein resulting in a decreased ability of cells to polymerize and reorganize actin, which is crucial for various cellular processes such as cellular division, immune synapse formation, phagocytosis, antigen presentation, and immune surveillance and regulation.
The immune dysfunction in WAS can lead to the development of other autoimmune diseases and non-Hodgkin lymphoma. Additionally, individuals with WAS have an elevated risk of developing chronic inflammatory bowel disease. Here, we discuss a patient with WAS and ulcerative colitis (UC) receiving vedolizumab (VDZ) who developed an Epstein Barr virus (EBV) associated mucocutaneous rectal ulcer.
Case Description/Methods: A 32-year-old male with a medical history of congenital WAS, with resulting splenectomy, and recurrent bacterial infections presented with bloody diarrhea and lower abdominal pain. After infectious causes were excluded, a colonoscopy revealed findings and pathology consistent with ulcerative colitis. Despite initial treatment with prednisone and sulfasalazine, he remained symptomatic. The patient was then started on VDZ, which resulted in a resolution of his symptoms and follow up colonoscopy showed normal mucosal and no further inflammation on pathology except for the presence of a new 15-cm rectal ulcer that tested positive for EBV; there was no dysplasia, granulomas, cytomegalovirus (CMV), human herpesvirus 8 (HHV-8), or lymphoma. The patient continued with VDZ treatment, and 6-month follow-up colonoscopy revealed a stable mucocutaneous ulcer (Figure 1). Rectal steroids were initiated and VDZ was discontinued, resulting in symptom resolution. The patient's condition has remained asymptomatic, and further evaluation is pending with a repeat colonoscopy.
Discussion: The presence of EBV-associated mucosal ulcers in immunosuppressed patient on VDZ raises several important questions regarding VDZ use in patients with WAS and UC. EBV-related mucosal ulcerations (EBVMCU) are commonly misdiagnosed under the lymphoma family. However, they are distinctly different and importantly share different prognoses. The precise role of VDZ in developing EBVMCU remains uncertain and future evaluation is warranted to understand the relationship between WAS, UC, and treatment with VDZ to guide and optimize management decisions.

Figure: Figure 1: Colonoscopy Identification of Ulcers in the Rectum
Disclosures:
Christopher Fernandes indicated no relevant financial relationships.
Christine Catinis indicated no relevant financial relationships.
Precious Anyanwu indicated no relevant financial relationships.
Timothy Ritter: Abbvie – Advisory Committee/Board Member, Speakers Bureau. ArdelyxArena – Advisory Committee/Board Member. Boehringer Ingelheim – Advisory Committee/Board Member. Bristol-Myers Squibb – Advisory Committee/Board Member, Speakers Bureau. Celgene – Advisory Committee/Board Member. Ferring – Advisory Committee/Board Member. Genentech/Roche Gilead – Advisory Committee/Board Member. Intercept – Advisory Committee/Board Member. Iterative Scopes – Advisory Committee/Board Member, Stock-publicly held company(excluding mutual/index funds). Janssen – Advisory Committee/Board Member, Speakers Bureau. Lilly – Advisory Committee/Board Member. Pfizer inc – Advisory Committee/Board Member, Speakers Bureau. Prometheus Biosciences – Advisory Committee/Board Member. Sanofi – Advisory Committee/Board Member. Takeda – Advisory Committee/Board Member, Speakers Bureau.
Christopher Fernandes, BSc1, Christine Catinis, MD, MS2, Precious Anyanwu, PharmD, PhD3, Timothy E.. Ritter, MD4. P2256 - Epstein-Barr Virus Associated Ulcers in a Patient with Ulcerative Colitis Taking Vedolizumab: A Case Report, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
