Monday Poster Session
Category: IBD
P2261 - De-Novo IBD After Kidney Transplant: Use of Vedolizumab Therapy
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
Has Audio

Zeel N. Modi, MD
University of Illinois-Chicago
Chicago, IL
Presenting Author(s)
Zeel N. Modi, MD1, Itishree Trivedi, MD, MS2
1University of Illinois-Chicago, Chicago, IL; 2University of Illinois at Chicago, Chicago, IL
Introduction: Solid organ transplant recipients often present with a variety of gastrointestinal (GI) complications from chronic immunosuppression and resultant intestinal dysbiosis with gut immune system dysregulation. New-onset inflammatory bowel disease (de-novo IBD) cases, rarely reported in kidney transplant (KT), are related to chronic immunosuppression. There is paucity of evidence-based management of post-KT de-novo IBD. We present a novel case of vedolizumab therapy use in post-KT de-novo IBD treatment and first case to report subsequent symptom relief.
Case Description/Methods: A 65-year-old female had KT in 2003 and remained stable on tacrolimus and prednisone. In 2021, she developed bloody diarrhea and abdominal pain requiring inpatient admission. C-reactive protein was 5.0 mg/L, and fecal calprotectin was 680 ug/g. Colonoscopy revealed sigmoid colon mucosal congestion and a cecal tubulovillous adenoma without dysplasia which was resected. Biopsies showed severe chronic inflammation with plasma-cell rich infiltrate with crypt architectural distortion and basal lympho-plasmacytosis. A diagnosis of de-novo IBD - indeterminate colitis was made. After initial improvement with mesalamine and prednisone, repeat colonoscopy noted mild persistent endoscopic inflammation with sigmoid erythema. At the prior cecal polypectomy site, residual polypoid tissue was resected with identification of high-grade dysplasia arising from a background of active chronic inflammation. After recurrent bloody diarrhea and biomarker elevation, treatment was advanced with vedolizumab improving clinical and biomarker response. Repeat colonoscopy, planned at 3-6 months, is pending.
Discussion: De-novo IBD post KT is a rare entity involving intestinal immune system dysregulation from chronic immunosuppression, with ~25 cases in the literature. Tacrolimus, in particular, increases risk of de-novo IBD by suppressing IL-2 production thereby reducing regulatory T-cells, which disrupts gut immunologic homeostasis. De-novo IBD after liver transplant is a well-recognized entity, where vedolizumab is positioned as a safe, steroid-sparing option given its gut selectivity and low risk of systemic infections or malignancies. However, there is a lack of treatment guidelines for post-KT de-novo IBD. The role of safe vedolizumab use in a post-KT de-novo IBD population is novel. Our patient had an inadequate response to mesalamine and developed high-grade dysplasia in an area of colitis, warranting prompt endoscopic remission of de-novo IBD.

Disclosures:
Zeel N. Modi, MD1, Itishree Trivedi, MD, MS2. P2261 - De-Novo IBD After Kidney Transplant: Use of Vedolizumab Therapy, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1University of Illinois-Chicago, Chicago, IL; 2University of Illinois at Chicago, Chicago, IL
Introduction: Solid organ transplant recipients often present with a variety of gastrointestinal (GI) complications from chronic immunosuppression and resultant intestinal dysbiosis with gut immune system dysregulation. New-onset inflammatory bowel disease (de-novo IBD) cases, rarely reported in kidney transplant (KT), are related to chronic immunosuppression. There is paucity of evidence-based management of post-KT de-novo IBD. We present a novel case of vedolizumab therapy use in post-KT de-novo IBD treatment and first case to report subsequent symptom relief.
Case Description/Methods: A 65-year-old female had KT in 2003 and remained stable on tacrolimus and prednisone. In 2021, she developed bloody diarrhea and abdominal pain requiring inpatient admission. C-reactive protein was 5.0 mg/L, and fecal calprotectin was 680 ug/g. Colonoscopy revealed sigmoid colon mucosal congestion and a cecal tubulovillous adenoma without dysplasia which was resected. Biopsies showed severe chronic inflammation with plasma-cell rich infiltrate with crypt architectural distortion and basal lympho-plasmacytosis. A diagnosis of de-novo IBD - indeterminate colitis was made. After initial improvement with mesalamine and prednisone, repeat colonoscopy noted mild persistent endoscopic inflammation with sigmoid erythema. At the prior cecal polypectomy site, residual polypoid tissue was resected with identification of high-grade dysplasia arising from a background of active chronic inflammation. After recurrent bloody diarrhea and biomarker elevation, treatment was advanced with vedolizumab improving clinical and biomarker response. Repeat colonoscopy, planned at 3-6 months, is pending.
Discussion: De-novo IBD post KT is a rare entity involving intestinal immune system dysregulation from chronic immunosuppression, with ~25 cases in the literature. Tacrolimus, in particular, increases risk of de-novo IBD by suppressing IL-2 production thereby reducing regulatory T-cells, which disrupts gut immunologic homeostasis. De-novo IBD after liver transplant is a well-recognized entity, where vedolizumab is positioned as a safe, steroid-sparing option given its gut selectivity and low risk of systemic infections or malignancies. However, there is a lack of treatment guidelines for post-KT de-novo IBD. The role of safe vedolizumab use in a post-KT de-novo IBD population is novel. Our patient had an inadequate response to mesalamine and developed high-grade dysplasia in an area of colitis, warranting prompt endoscopic remission of de-novo IBD.

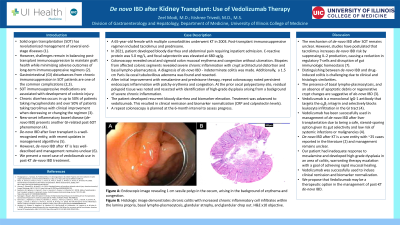
Figure: Figure 1: (A) Endoscopic image from repeat colonoscopy revealing 0.99 mm sessile polyp in the cecum. (B) Histologic image demonstrating ongoing chronic colitis showing increased chronic cell infiltrates within the lamina propria, basal lympho-plasmacytosis, glandular atrophy, and glandular drop out. H&E x10 objective.
Disclosures:
Zeel Modi indicated no relevant financial relationships.
Itishree Trivedi indicated no relevant financial relationships.
Zeel N. Modi, MD1, Itishree Trivedi, MD, MS2. P2261 - De-Novo IBD After Kidney Transplant: Use of Vedolizumab Therapy, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
