Monday Poster Session
Category: Liver
P2468 - A Rare Case of HBV Reactivation With Delayed Cholestatic Effect Secondary to Acalabrutinib
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
Has Audio
- AS
Abdullah S. Shaikh, MD
University of Texas MD Anderson Cancer Center
Houston, TX
Presenting Author(s)
Award: Presidential Poster Award
Abdullah S. Shaikh, MD, Susan Abraham, MD, Hao Chi Zhang, MD
University of Texas MD Anderson Cancer Center, Houston, TX
Introduction: Hepatitis B virus reactivation (HBVr) is described by return of HBV viremia in patients with resolved HBV or an increase in HBV viremia in patients with inactive chronic HBV. HBVr can occur with use of immunosuppressants such as corticosteroids, rituximab, anthracyclines, cyclophosphamide and vinca alkaloids. We present a rare case of HBVr in a patient with HBV exposure with negative HBsAg while on tyrosine kinase inhibitor acalbrutinib.
Case Description/Methods: A 66-year-old male with recurrent mantle cell lymphoma (MCL) and positive HBcAb but negative HBsAg and HBV DNA, implying prior exposure without active infection, was started on venetoclax and acalabrutinib without entecavir prophylaxis. His prior MCL was successfully treated with rituximab, cyclophosphamide, vincristine sulfate, doxorubicin, methotrexate, and cytarabine 5 years prior; he received HBV prophylaxis with entecavir for 1 year after completion of chemotherapy. He developed HBVr 1.5 years after his recurrent MCL was diagnosed. Entecavir was initiated but liver enzymes worsened with a delayed rise in bilirubin. Serologic work-up for liver disease was unrevealing except for weakly positive anti-smooth muscle and anti-mitochondrial antibodies. Abdomen MRI/MRCP showed no biliary dilatation. The delayed rise in bilirubin was concerning for hepatic dysfunction, specifically drug induced liver injury related to a concurrent autoimmune-like process. Steroid therapy was considered but the patient’s INR and mental status remained normal. Liver biopsy showed panacinar hepatitis with mild steatosis; there was no lymphoma involvement or advanced fibrosis. The biopsy was consistent with HBVr which was deemed secondary to acalabrutinib. Over time, his bilirubin and HBV viral load normalized on long-term entecavir, revealing he had a delayed cholestatic effect.
Discussion: The risk of HBVr is highest in patients receiving hematopoietic stem cell transplants and B cell-depleting therapies. Patients with HBsAg-positive status are at higher risk for HBVr, but patients with HBV exposure without active viremia also bear risk. There have been rare cases of HBVr with concurrent acalbrutinib and other immunosuppressant use. It is also hypothesized that venetoclax could cause HBVr but there are no clinical reports. The exact incidence is not known for either drug. In conclusion, our case highlights the risk of HBVr in patients previously exposed to HBV without active infection and are receiving lower risk immunosuppressants such as acalbrutinib.

Disclosures:
Abdullah S. Shaikh, MD, Susan Abraham, MD, Hao Chi Zhang, MD. P2468 - A Rare Case of HBV Reactivation With Delayed Cholestatic Effect Secondary to Acalabrutinib, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
Abdullah S. Shaikh, MD, Susan Abraham, MD, Hao Chi Zhang, MD
University of Texas MD Anderson Cancer Center, Houston, TX
Introduction: Hepatitis B virus reactivation (HBVr) is described by return of HBV viremia in patients with resolved HBV or an increase in HBV viremia in patients with inactive chronic HBV. HBVr can occur with use of immunosuppressants such as corticosteroids, rituximab, anthracyclines, cyclophosphamide and vinca alkaloids. We present a rare case of HBVr in a patient with HBV exposure with negative HBsAg while on tyrosine kinase inhibitor acalbrutinib.
Case Description/Methods: A 66-year-old male with recurrent mantle cell lymphoma (MCL) and positive HBcAb but negative HBsAg and HBV DNA, implying prior exposure without active infection, was started on venetoclax and acalabrutinib without entecavir prophylaxis. His prior MCL was successfully treated with rituximab, cyclophosphamide, vincristine sulfate, doxorubicin, methotrexate, and cytarabine 5 years prior; he received HBV prophylaxis with entecavir for 1 year after completion of chemotherapy. He developed HBVr 1.5 years after his recurrent MCL was diagnosed. Entecavir was initiated but liver enzymes worsened with a delayed rise in bilirubin. Serologic work-up for liver disease was unrevealing except for weakly positive anti-smooth muscle and anti-mitochondrial antibodies. Abdomen MRI/MRCP showed no biliary dilatation. The delayed rise in bilirubin was concerning for hepatic dysfunction, specifically drug induced liver injury related to a concurrent autoimmune-like process. Steroid therapy was considered but the patient’s INR and mental status remained normal. Liver biopsy showed panacinar hepatitis with mild steatosis; there was no lymphoma involvement or advanced fibrosis. The biopsy was consistent with HBVr which was deemed secondary to acalabrutinib. Over time, his bilirubin and HBV viral load normalized on long-term entecavir, revealing he had a delayed cholestatic effect.
Discussion: The risk of HBVr is highest in patients receiving hematopoietic stem cell transplants and B cell-depleting therapies. Patients with HBsAg-positive status are at higher risk for HBVr, but patients with HBV exposure without active viremia also bear risk. There have been rare cases of HBVr with concurrent acalbrutinib and other immunosuppressant use. It is also hypothesized that venetoclax could cause HBVr but there are no clinical reports. The exact incidence is not known for either drug. In conclusion, our case highlights the risk of HBVr in patients previously exposed to HBV without active infection and are receiving lower risk immunosuppressants such as acalbrutinib.

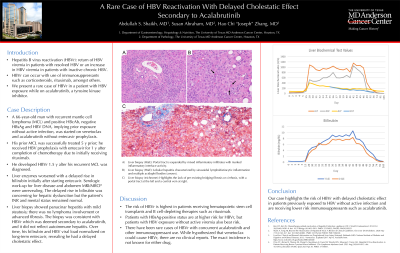
Figure: A) Portal tract is expanded by mixed inflammatory infiltrates with marked inflammatory interface activity. B) Lobular hepatitis characterized by sinusoidal lymphohistiocytic inflammation and multiple acidophil bodies (arrows). C) Trichrome stain highlights the lack of pre-existing bridging fibrosis or cirrhosis, with a portal tract at the left and a central vein at right. D) Liver biochemical lab work (ALP = alkaline phosphatase, T bili = total bilirubin, D bili = direct bilirubin) over time. INR (not graphed) remained within normal limits throughout the entire HBV reactivation episode.
Disclosures:
Abdullah Shaikh indicated no relevant financial relationships.
Susan Abraham indicated no relevant financial relationships.
Hao Chi Zhang indicated no relevant financial relationships.
Abdullah S. Shaikh, MD, Susan Abraham, MD, Hao Chi Zhang, MD. P2468 - A Rare Case of HBV Reactivation With Delayed Cholestatic Effect Secondary to Acalabrutinib, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.

