Monday Poster Session
Category: Liver
P2588 - Drug-Induced Liver Injury Secondary to Escitalopram
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
Has Audio
- TR
Tabeer Rana, DO
Allegheny Health Network
Pittsburgh, PA
Presenting Author(s)
Tabeer Rana, DO1, James Rock, DO1, Alexandra Johnston, DO1, Palgun Nisarga, MBBS2, Mark Bunker, MD1
1Allegheny Health Network, Pittsburgh, PA; 2AGH, Pittsburgh, PA
Introduction: Escitalopram (ESC) is a selective serotonin reuptake inhibitor (SSRI) routinely prescribed as a first-line antidepressant. Hepatotoxicity is an exceedingly rare side effect of ESC (< 1%), primarily limited to case reports. We present a rare case of drug induced liver injury (DILI) secondary to ESC.
Case Description/Methods: 62 y.o F with pmhx of hepatic steatosis, depression (on ESC since 2007), and obesity class I (BMI 33.6) was referred to a gastroenterologist for chronic transaminase elevations. She endorsed improving fatigue and myalgias that she associated with “long coronavirus-19” from 04/2020. She denied hx of or risk factors for viral hepatitis, familial liver disease, or alcohol use. She reported moderate weight gain during the coronavirus-19 pandemic that did not precede initial lab abnormalities.
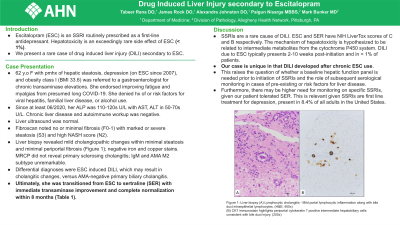
Since at least 06/2020, her ALP was 110-120s U/L with AST, ALT in 50-70s U/L. Chronic liver disease and autoimmune workup was negative. Liver ultrasound was normal. Fibroscan noted no or minimal fibrosis (F0-1) with, of note, marked or severe steatosis (S3) and high NASH score (N2). Liver biopsy revealed mild cholangiopathic changes within minimal steatosis and minimal periportal fibrosis (Figure 1); negative iron and copper stains. MRCP did not reveal primary sclerosing cholangitis; IgM and AMA M2 subtype unremarkable. Differential diagnoses considered were ESC induced DILI, which may result in cholangitic changes, versus AMA-negative primary biliary cholangitis. Ultimately, she was transitioned from ESC to sertraline (SER) with immediate transaminase improvement and ultimately complete normalization within 8 months (Table 1).
Discussion: SSRIs are a rare cause of DILI. ESC and SER have NIH LiverTox scores of C and B respectively. The mechanism of hepatotoxicity is unknown but hypothesized to be related to intermediate metabolites from the cytochrome P450 system. Of note, DILI due to ESC typically presents 2-10 weeks post-initiation and in < 1% of patients. Our case is unique in that DILI developed after chronic ESC use. This raises the question of whether a baseline hepatic function panel is needed prior to initiation of SSRIs and the role of subsequent serological monitoring in cases of pre-existing or risk factors for development of liver disease. Furthermore, there may be higher need for monitoring on specific SSRIs, given our patient tolerated SER. This is relevant given SSRIs are first line treatment for depression, present in 8.4% of all adults in the United States.

Disclosures:
Tabeer Rana, DO1, James Rock, DO1, Alexandra Johnston, DO1, Palgun Nisarga, MBBS2, Mark Bunker, MD1. P2588 - Drug-Induced Liver Injury Secondary to Escitalopram, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Allegheny Health Network, Pittsburgh, PA; 2AGH, Pittsburgh, PA
Introduction: Escitalopram (ESC) is a selective serotonin reuptake inhibitor (SSRI) routinely prescribed as a first-line antidepressant. Hepatotoxicity is an exceedingly rare side effect of ESC (< 1%), primarily limited to case reports. We present a rare case of drug induced liver injury (DILI) secondary to ESC.
Case Description/Methods: 62 y.o F with pmhx of hepatic steatosis, depression (on ESC since 2007), and obesity class I (BMI 33.6) was referred to a gastroenterologist for chronic transaminase elevations. She endorsed improving fatigue and myalgias that she associated with “long coronavirus-19” from 04/2020. She denied hx of or risk factors for viral hepatitis, familial liver disease, or alcohol use. She reported moderate weight gain during the coronavirus-19 pandemic that did not precede initial lab abnormalities.
Since at least 06/2020, her ALP was 110-120s U/L with AST, ALT in 50-70s U/L. Chronic liver disease and autoimmune workup was negative. Liver ultrasound was normal. Fibroscan noted no or minimal fibrosis (F0-1) with, of note, marked or severe steatosis (S3) and high NASH score (N2). Liver biopsy revealed mild cholangiopathic changes within minimal steatosis and minimal periportal fibrosis (Figure 1); negative iron and copper stains. MRCP did not reveal primary sclerosing cholangitis; IgM and AMA M2 subtype unremarkable. Differential diagnoses considered were ESC induced DILI, which may result in cholangitic changes, versus AMA-negative primary biliary cholangitis. Ultimately, she was transitioned from ESC to sertraline (SER) with immediate transaminase improvement and ultimately complete normalization within 8 months (Table 1).
Discussion: SSRIs are a rare cause of DILI. ESC and SER have NIH LiverTox scores of C and B respectively. The mechanism of hepatotoxicity is unknown but hypothesized to be related to intermediate metabolites from the cytochrome P450 system. Of note, DILI due to ESC typically presents 2-10 weeks post-initiation and in < 1% of patients. Our case is unique in that DILI developed after chronic ESC use. This raises the question of whether a baseline hepatic function panel is needed prior to initiation of SSRIs and the role of subsequent serological monitoring in cases of pre-existing or risk factors for development of liver disease. Furthermore, there may be higher need for monitoring on specific SSRIs, given our patient tolerated SER. This is relevant given SSRIs are first line treatment for depression, present in 8.4% of all adults in the United States.

Figure: Figure 1: Liver biopsy (A) Lymphocytic cholangitis - Mild portal lymphocytic inflammation along with bile duct intraepithelial lymphocytes. (H&E; 400x) (B) CK7 immunostain highlights periportal cytokeratin 7 positive intermediate hepatobiliary cells consistent with bile duct injury. (200x)
Disclosures:
Tabeer Rana indicated no relevant financial relationships.
James Rock indicated no relevant financial relationships.
Alexandra Johnston indicated no relevant financial relationships.
Palgun Nisarga indicated no relevant financial relationships.
Mark Bunker indicated no relevant financial relationships.
Tabeer Rana, DO1, James Rock, DO1, Alexandra Johnston, DO1, Palgun Nisarga, MBBS2, Mark Bunker, MD1. P2588 - Drug-Induced Liver Injury Secondary to Escitalopram, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
