Monday Poster Session
Category: Small Intestine
P2630 - Comparison of an At-Home Hydrogen Methane Breath Analysis Device With Reference Benchtop Devices
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
Has Audio
- BM
Barry McBride, MSc
FoodMarble
Dublin, Dublin, Ireland
Presenting Author(s)
Barry McBride, MSc1, Eoghan Lafferty, MSc1, Catherine Waechter, MSc1, Claire Shortt, PhD2, Peter Harte, PhD1, Aonghus Shortt, PhD2, Guillermo Barahona, MD, MBA3, Pankaj Pasricha, MD, MBBS3
1FoodMarble, Dublin, Dublin, Ireland; 2FoodMarble Digestive Health Ltd, Dublin, Dublin, Ireland; 3Mayo Clinic Arizona, Scottsdale, AZ
Introduction: Breath analysis offers simple, non-invasive testing that’s relevant for a range of digestive disorders. However, it hasn’t previously been practical to perform repeat testing to assess treatment response. To this end, an at-home app-connected H2-CH4 breath analysis device has been compared to two reference benchtop devices, to assess equivalence, by performing lactulose breath tests.
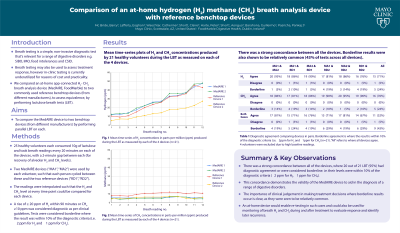
Methods: 25 healthy volunteers each consumed 10g of lactulose and took breath readings every 20 minutes on each of the devices, with a 2-minute gap between each (for recovery of alveolar H2 and CH4 levels). Two app-connected devices (“ACD1”, “ACD2”) were used by each volunteer, such that each person cycled between these and the two reference devices (“RD1”, “RD2”). The readings were interpolated such that the H2 and CH4 level at every time point could be compared for each device. This enabled the calculation of the rise in H2 within 90 minutes of ingesting the substrate for each device. A rise of ≥ 20ppm of H2 within 90 minutes or CH4 ≥ 10ppm was considered diagnostic as per clinical guidelines.
Results: 4 volunteers were excluded due to high baseline readings. 20 of the remaining 21 (95%) had diagnostic agreement or were considered borderline, i.e. their levels were within 10% of the diagnostic criteria (± 2ppm for H2, ± 1ppm for CH4). The single disagreement was where RD1 was 2.6ppm above the criteria for H2. When comparing pairs of devices, the highest diagnostic agreement for H2 was between ACD1 and ACD2 (1 borderline case, remainder in agreement). The lowest pair-wise agreement was for RD1 and RD2, which disagreed in one case and the results were borderline in 4 cases. For CH4 diagnostic agreement, ACD2 and RD1 agreed in all cases except a single borderline case, whereas the lowest rate of agreement occurred for ACD1 and the RD2, which had 4 borderline cases, with the remainder in agreement. Considering H2 and CH4, ACD1 and ACD2 had the most diagnostic agreement with 4 borderlines cases, whereas RD1 and RD2 had the lowest with 1 disagreement and 6 borderline cases.
Discussion: There was very strong concordance between all of the devices. The importance of clinical judgement in making treatment decisions where borderline results occur is clear, as they were seen to be relatively common. An at-home device would enable re-testing in such cases and could also be used for monitoring of breath H2 and CH4 during and after treatment to evaluate response and identify later recurrence.

Disclosures:
Barry McBride, MSc1, Eoghan Lafferty, MSc1, Catherine Waechter, MSc1, Claire Shortt, PhD2, Peter Harte, PhD1, Aonghus Shortt, PhD2, Guillermo Barahona, MD, MBA3, Pankaj Pasricha, MD, MBBS3. P2630 - Comparison of an At-Home Hydrogen Methane Breath Analysis Device With Reference Benchtop Devices, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1FoodMarble, Dublin, Dublin, Ireland; 2FoodMarble Digestive Health Ltd, Dublin, Dublin, Ireland; 3Mayo Clinic Arizona, Scottsdale, AZ
Introduction: Breath analysis offers simple, non-invasive testing that’s relevant for a range of digestive disorders. However, it hasn’t previously been practical to perform repeat testing to assess treatment response. To this end, an at-home app-connected H2-CH4 breath analysis device has been compared to two reference benchtop devices, to assess equivalence, by performing lactulose breath tests.
Methods: 25 healthy volunteers each consumed 10g of lactulose and took breath readings every 20 minutes on each of the devices, with a 2-minute gap between each (for recovery of alveolar H2 and CH4 levels). Two app-connected devices (“ACD1”, “ACD2”) were used by each volunteer, such that each person cycled between these and the two reference devices (“RD1”, “RD2”). The readings were interpolated such that the H2 and CH4 level at every time point could be compared for each device. This enabled the calculation of the rise in H2 within 90 minutes of ingesting the substrate for each device. A rise of ≥ 20ppm of H2 within 90 minutes or CH4 ≥ 10ppm was considered diagnostic as per clinical guidelines.
Results: 4 volunteers were excluded due to high baseline readings. 20 of the remaining 21 (95%) had diagnostic agreement or were considered borderline, i.e. their levels were within 10% of the diagnostic criteria (± 2ppm for H2, ± 1ppm for CH4). The single disagreement was where RD1 was 2.6ppm above the criteria for H2. When comparing pairs of devices, the highest diagnostic agreement for H2 was between ACD1 and ACD2 (1 borderline case, remainder in agreement). The lowest pair-wise agreement was for RD1 and RD2, which disagreed in one case and the results were borderline in 4 cases. For CH4 diagnostic agreement, ACD2 and RD1 agreed in all cases except a single borderline case, whereas the lowest rate of agreement occurred for ACD1 and the RD2, which had 4 borderline cases, with the remainder in agreement. Considering H2 and CH4, ACD1 and ACD2 had the most diagnostic agreement with 4 borderlines cases, whereas RD1 and RD2 had the lowest with 1 disagreement and 6 borderline cases.
Discussion: There was very strong concordance between all of the devices. The importance of clinical judgement in making treatment decisions where borderline results occur is clear, as they were seen to be relatively common. An at-home device would enable re-testing in such cases and could also be used for monitoring of breath H2 and CH4 during and after treatment to evaluate response and identify later recurrence.

Figure: Example of one of the lactulose breath tests, as performed by one of the volunteers. Time-series of hydrogen and methane concentrations in parts-per-million (ppm) as measured by each of the 4 devices is shown. The linearly interpolated values are indicated by the connecting lines with the values at 90 minutes post-ingestion of the substrate marked for clarity.
Disclosures:
Barry McBride: FoodMarble Digestive Health Ltd – Employee.
Eoghan Lafferty: FoodMarble Digestive Health Ltd – Employee.
Catherine Waechter: FoodMarble Digestive Health Ltd – Employee.
Claire Shortt: FoodMarble Digestive Health – Employee. FoodMarble Digestive Health – Employee.
Peter Harte: FoodMarble Digestive Health Ltd – Employee, Owner/Ownership Interest.
Aonghus Shortt: FoodMarble Digestive Health Ltd – Employee, Owner/Ownership Interest.
Guillermo Barahona indicated no relevant financial relationships.
Pankaj Pasricha: FoodMarble – Consultant. Neurogastrx – Consultant. Vanda – Consultant.
Barry McBride, MSc1, Eoghan Lafferty, MSc1, Catherine Waechter, MSc1, Claire Shortt, PhD2, Peter Harte, PhD1, Aonghus Shortt, PhD2, Guillermo Barahona, MD, MBA3, Pankaj Pasricha, MD, MBBS3. P2630 - Comparison of an At-Home Hydrogen Methane Breath Analysis Device With Reference Benchtop Devices, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
