Tuesday Poster Session
Category: Stomach
P4186A - An Adaptive Phase 2/3 Study Design to Investigate the Efficacy and Safety of Dupilumab Therapy Compared With Placebo in Adults and Adolescents With Eosinophilic Gastritis With or Without Eosinophilic Duodenitis
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
Has Audio
- ED
Evan S. Dellon, MD, MPH
University of North Carolina School of Medicine
Chapel Hill, North Carolina
Presenting Author(s)
Evan S. Dellon, MD, MPH1, Marc E. Rothenberg, MD, PhD2, Mirna Chehade, MD, MPH3, Eric Mortensen, MD, PhD4, Elizabeth Laws, PhD5, Jennifer Maloney, MD4, Renata Martincova, MD5, Heather Paleczny, MD, PhD4, Lila Glotfelty, MD, PhD5, Arsalan Shabbir, MD, PhD4
1University of North Carolina School of Medicine, Chapel Hill, NC; 2Cincinnati Children’s Hospital Medical Center and University of Cincinnati College of Medicine, Cincinnati, OH; 3Mount Sinai Center for Eosinophilic Disorders, Icahn School of Medicine at Mount Sinai, New York, NY; 4Regeneron Pharmaceuticals Inc., Tarrytown, NY; 5Sanofi, Bridgewater, NJ
Introduction: Eosinophilic gastritis (EoG) and eosinophilic duodenitis (EoD) are chronic inflammatory diseases characterized by pathologic levels of eosinophils (eos) in the stomach and duodenum, respectively. Common symptoms include abdominal pain, nausea, vomiting, early satiety, and weight loss; patients may progress to severe malabsorption and malnutrition. Evidence suggests that type 2 cell cytokines (IL-4, IL-5, and IL-13) play a role in EoG pathogenesis. Currently, there are no approved therapies for EoG. Dupilumab, a fully human monoclonal antibody, blocks the shared receptor component for IL-4 and IL-13, key drivers of type 2 inflammation in multiple diseases, including eosinophilic esophagitis. Herein, we present the phase 2/3 ENGAGE (NCT05831176) study design which will investigate the efficacy and safety of dupilumab vs placebo in adult and adolescent patients with EoG with or without EoD.
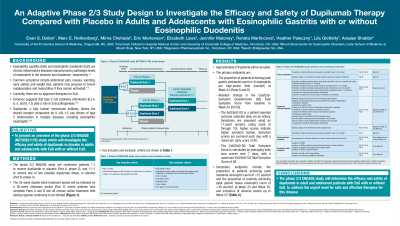
Methods: This study will randomize patients 1:1 to receive dupilumab or placebo (Part A, phase 2), and 1:1:1 to receive one of two possible dupilumab doses, or placebo (Part B, phase 3). Following this will be a 28-week extension period (Part C) where patients who complete Parts A and B will receive active treatment (but dosing regimen will be masked) (Figure). Key inclusion criteria include age ≥12 years, documented diagnosis of EoG by biopsy (≥3 months prior to screening), and history of ≥2 episodes of EoG symptoms per week. Key exclusion criteria include body weight < 40 kg, esophageal stricture unable to be passed with standard upper endoscope or esophageal stricture that requires dilation, Crohn’s disease, eosinophilic colitis, ulcerative colitis, celiac disease, or prior gastric or duodenal surgery.
Results: Primary endpoints will include proportion of patients achieving peak gastric eosinophil count of ≤6 eosinophils per high-power field (eos/hpf) at Week 24 (Parts A and B) and absolute change in the EoG/EoD Symptom Questionnaire Total Symptom Score from baseline to Week 24 (Part B). Secondary endpoints will include proportion of patients achieving both peak gastric and duodenal eos of ≤6 eos/hpf and ≤15 eos/hpf, respectively at Week 24 and Week 52, and incidence of adverse events up to Week 52 (Table).
Discussion: The phase 2/3 ENGAGE study will determine the efficacy and safety of dupilumab in adult and adolescent patients with EoG with or without EoD, to address the urgent need for safe and effective therapies for this disease.

Disclosures:
Evan S. Dellon, MD, MPH1, Marc E. Rothenberg, MD, PhD2, Mirna Chehade, MD, MPH3, Eric Mortensen, MD, PhD4, Elizabeth Laws, PhD5, Jennifer Maloney, MD4, Renata Martincova, MD5, Heather Paleczny, MD, PhD4, Lila Glotfelty, MD, PhD5, Arsalan Shabbir, MD, PhD4. P4186A - An Adaptive Phase 2/3 Study Design to Investigate the Efficacy and Safety of Dupilumab Therapy Compared With Placebo in Adults and Adolescents With Eosinophilic Gastritis With or Without Eosinophilic Duodenitis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1University of North Carolina School of Medicine, Chapel Hill, NC; 2Cincinnati Children’s Hospital Medical Center and University of Cincinnati College of Medicine, Cincinnati, OH; 3Mount Sinai Center for Eosinophilic Disorders, Icahn School of Medicine at Mount Sinai, New York, NY; 4Regeneron Pharmaceuticals Inc., Tarrytown, NY; 5Sanofi, Bridgewater, NJ
Introduction: Eosinophilic gastritis (EoG) and eosinophilic duodenitis (EoD) are chronic inflammatory diseases characterized by pathologic levels of eosinophils (eos) in the stomach and duodenum, respectively. Common symptoms include abdominal pain, nausea, vomiting, early satiety, and weight loss; patients may progress to severe malabsorption and malnutrition. Evidence suggests that type 2 cell cytokines (IL-4, IL-5, and IL-13) play a role in EoG pathogenesis. Currently, there are no approved therapies for EoG. Dupilumab, a fully human monoclonal antibody, blocks the shared receptor component for IL-4 and IL-13, key drivers of type 2 inflammation in multiple diseases, including eosinophilic esophagitis. Herein, we present the phase 2/3 ENGAGE (NCT05831176) study design which will investigate the efficacy and safety of dupilumab vs placebo in adult and adolescent patients with EoG with or without EoD.
Methods: This study will randomize patients 1:1 to receive dupilumab or placebo (Part A, phase 2), and 1:1:1 to receive one of two possible dupilumab doses, or placebo (Part B, phase 3). Following this will be a 28-week extension period (Part C) where patients who complete Parts A and B will receive active treatment (but dosing regimen will be masked) (Figure). Key inclusion criteria include age ≥12 years, documented diagnosis of EoG by biopsy (≥3 months prior to screening), and history of ≥2 episodes of EoG symptoms per week. Key exclusion criteria include body weight < 40 kg, esophageal stricture unable to be passed with standard upper endoscope or esophageal stricture that requires dilation, Crohn’s disease, eosinophilic colitis, ulcerative colitis, celiac disease, or prior gastric or duodenal surgery.
Results: Primary endpoints will include proportion of patients achieving peak gastric eosinophil count of ≤6 eosinophils per high-power field (eos/hpf) at Week 24 (Parts A and B) and absolute change in the EoG/EoD Symptom Questionnaire Total Symptom Score from baseline to Week 24 (Part B). Secondary endpoints will include proportion of patients achieving both peak gastric and duodenal eos of ≤6 eos/hpf and ≤15 eos/hpf, respectively at Week 24 and Week 52, and incidence of adverse events up to Week 52 (Table).
Discussion: The phase 2/3 ENGAGE study will determine the efficacy and safety of dupilumab in adult and adolescent patients with EoG with or without EoD, to address the urgent need for safe and effective therapies for this disease.

Figure: Figure. Phase 2/3 ENGAGE study (NCT05831176)
*Enrollment for adolescents is ex-US in Part A. §Calculated using data from the EoG/EoD-SQ eDiary. †On a scale of 1–10. ‡Patients who received placebo in Parts A and B will be randomized 1:1.
EoD, eosinophilic duodenitis; EoG, eosinophilic gastritis; R, randomized; SQ, symptom questionnaire
*Enrollment for adolescents is ex-US in Part A. §Calculated using data from the EoG/EoD-SQ eDiary. †On a scale of 1–10. ‡Patients who received placebo in Parts A and B will be randomized 1:1.
EoD, eosinophilic duodenitis; EoG, eosinophilic gastritis; R, randomized; SQ, symptom questionnaire
Disclosures:
Evan Dellon: Abbott – Consultant. Abbvie – Consultant. Adare/Ellodi – Consultant, Grant/Research Support. Aimmune – Consultant. Akesobio – Consultant. Alfasigma – Consultant. ALK – Consultant. Allakos – Consultant, Grant/Research Support. Amgen – Consultant. Aqilion – Consultant. Arena/Pfizer – Consultant, Grant/Research Support. Aslan – Consultant. AstraZeneca – Consultant, Grant/Research Support. Avir – Consultant. Banner Pharmaceuticals – Grant/Research Support. Biorasi – Consultant. Calypso – Consultant. Celgene/Receptos/BMS – Consultant, Grant/Research Support. Celldex – Consultant. Eli Lilly – Consultant. EsoCap – Consultant. Eupraxia – Consultant. Ferring – Consultant. Gossamer Bio – Consultant. GSK – Consultant, Grant/Research Support. Holoclara – Consultant, Grant/Research Support. Invea – Consultant, Grant/Research Support. Knightpoint – Consultant. Landos – Consultant. LucidDx – Consultant. Meritage – Grant/Research Support. Miraca – Grant/Research Support. Morphic – Consultant. Nexstone Immunology – Consultant. Nutricia – Consultant, Grant/Research Support. Parexel/Calyx – Consultant. Phathom – Consultant. Regeneron Pharmaceuticals Inc. – Consultant, Grant/Research Support. Revolo Biotherapeutics – Consultant, Grant/Research Support. Robarts/Alimentiv – Consultant. Salix – Consultant. Sanofi – Consultant. Shire/Takeda – Consultant, Grant/Research Support. Target RWE – Consultant. Upstream Bio – Consultant.
Marc Rothenberg: Allakos – Consultant. AstraZeneca – Consultant. BMS – Consultant. Celldex – Consultant, Equity interest. ClostraBio – Consultant, Equity interest. Ellodi Pharmaceuticals – Consultant. GSK – Consultant. Guidepoint – Consultant. Mapi Research Trust – Royalties. Nextstone One – Consultant, Equity interest. PulmOne – Consultant, Equity interest. Regeneron Pharmaceuticals Inc. – Consultant. Revolo – Consultant. Sanofi – Consultant. Santa Ana Bio – Consultant, Equity interest. Serpin Pharma – Consultant, Equity interest. Spoon Guru – Consultant, Equity interest. Teva Pharmaceuticals – Royalties. UpToDate – Royalties.
Mirna Chehade: Adare/Ellodi – Consultant, Grant/Research Support. Allakos – Consultant, Grant/Research Support. AstraZeneca – Consultant, Grant/Research Support. BMS – Consultant. Danone – Grant/Research Support. Nexstone Immunology – Consultant. Phathom – Consultant. Recludix – Consultant. Regeneron Pharmaceuticals Inc. – Consultant, Grant/Research Support. Sanofi – Consultant. Shire/Takeda – Consultant, Grant/Research Support.
Eric Mortensen: Regeneron Pharmaceuticals Inc. – Employee, Shareholder.
Elizabeth Laws: Sanofi – Employee, Stock Options.
Jennifer Maloney: Regeneron Pharmaceuticals Inc. – Employee, Shareholder.
Renata Martincova: Sanofi – Employee, Stock Options.
Heather Paleczny: Regeneron Pharmaceuticals Inc. – Employee, Shareholder.
Lila Glotfelty: Sanofi – Employee, Stock Options.
Arsalan Shabbir: Regeneron Pharmaceuticals Inc. – Employee, Stock Options.
Evan S. Dellon, MD, MPH1, Marc E. Rothenberg, MD, PhD2, Mirna Chehade, MD, MPH3, Eric Mortensen, MD, PhD4, Elizabeth Laws, PhD5, Jennifer Maloney, MD4, Renata Martincova, MD5, Heather Paleczny, MD, PhD4, Lila Glotfelty, MD, PhD5, Arsalan Shabbir, MD, PhD4. P4186A - An Adaptive Phase 2/3 Study Design to Investigate the Efficacy and Safety of Dupilumab Therapy Compared With Placebo in Adults and Adolescents With Eosinophilic Gastritis With or Without Eosinophilic Duodenitis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
