Tuesday Poster Session
Category: Interventional Endoscopy
P3732 - Safe Endoscopic Removal of Unbranded, Internationally Placed Intra-Gastric Balloons Utilizing Regularly Available Endoscopic Tools
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
.jpg)
Murad H. Ali, MD
University at Buffalo
Buffalo, NY
Presenting Author(s)
Murad H. Ali, MD1, Rabia de Latour, MD2
1University at Buffalo, Buffalo, NY; 2NYU Grossman School of Medicine, New York, NY
Introduction: Intragastric balloons (IGB) are space occupying weight loss devices meant for short term use (6 months) after which endoscopic removal can be achieved with a dedicated removal device. Most public hospitals do not offer placement or removal due to lack of coverage and inability of patients to self-pay for this procedure. We present cases of patients who presented to Bellevue Hospital, with non-FDA approved IGB placed internationally.
Case Description/Methods: Case 1: 38 year old obese M with IGB placement in Colombia 10 days prior was admitted for presyncope and melena.
Case 2: 49 year old obese F with IGB placement in Colombia 1 year prior was admitted for abdominal pain for 2 weeks.
Case 3: 25 year old transgender F with IGB placement 2 years prior in Thailand, admitted for epigastric pain and nausea for 5 days.
Case 4 : 32 year old obese F with IGB placement in the Dominican Republic (DR) one year prior presented to bariatric surgery clinic requesting surgical intervention due to lack of weight loss and were referred for outpatient endoscopic removal of IGB.
Case 5: 44 year old obese M with IGB placement in Dominican Republic 3 months prior. Referred from PCP for removal due to poor tolerance, nausea and vomiting.
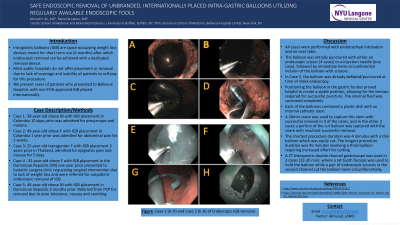
Discussion: All cases were performed with endotracheal intubation and no overtube. The balloon was initially punctured with either an endoscopic scissor (4 cases) or a injection needle (one case), followed by immediate hemi-circumfirential incision of the balloon with scissors. In Case 5, the balloon was already deflated/punctured at time of index endoscopy. Positioning the balloon in the gastric fundus proved helpful to create a stable position, allowing for the tension required for successful puncture. The internal fluid was suctioned completely. Each of the balloons contained a plastic disk with an internal catheter stem. A 20mm snare was used to capture this stem with successful removal in 3 of the cases, and in the other 2 cases a portion of the cut balloon was captured with the snare with resultant successful removal. The shortest procedure duration was 4 minutes with a thin balloon which was easily cut. The longest procedure duration was 45 minutes involving a thick balloon requiring increased effort for cutting. A 2T therapeutic double channel gastroscope was used in 3 cases (22-30 min), where a rat tooth forceps was used to hold the balloon while a pair of endoscopic scissors in the second channel cut the balloon hemi-circumferentially.

Disclosures:
Murad H. Ali, MD1, Rabia de Latour, MD2. P3732 - Safe Endoscopic Removal of Unbranded, Internationally Placed Intra-Gastric Balloons Utilizing Regularly Available Endoscopic Tools, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1University at Buffalo, Buffalo, NY; 2NYU Grossman School of Medicine, New York, NY
Introduction: Intragastric balloons (IGB) are space occupying weight loss devices meant for short term use (6 months) after which endoscopic removal can be achieved with a dedicated removal device. Most public hospitals do not offer placement or removal due to lack of coverage and inability of patients to self-pay for this procedure. We present cases of patients who presented to Bellevue Hospital, with non-FDA approved IGB placed internationally.
Case Description/Methods: Case 1: 38 year old obese M with IGB placement in Colombia 10 days prior was admitted for presyncope and melena.
Case 2: 49 year old obese F with IGB placement in Colombia 1 year prior was admitted for abdominal pain for 2 weeks.
Case 3: 25 year old transgender F with IGB placement 2 years prior in Thailand, admitted for epigastric pain and nausea for 5 days.
Case 4 : 32 year old obese F with IGB placement in the Dominican Republic (DR) one year prior presented to bariatric surgery clinic requesting surgical intervention due to lack of weight loss and were referred for outpatient endoscopic removal of IGB.
Case 5: 44 year old obese M with IGB placement in Dominican Republic 3 months prior. Referred from PCP for removal due to poor tolerance, nausea and vomiting.
Discussion: All cases were performed with endotracheal intubation and no overtube. The balloon was initially punctured with either an endoscopic scissor (4 cases) or a injection needle (one case), followed by immediate hemi-circumfirential incision of the balloon with scissors. In Case 5, the balloon was already deflated/punctured at time of index endoscopy. Positioning the balloon in the gastric fundus proved helpful to create a stable position, allowing for the tension required for successful puncture. The internal fluid was suctioned completely. Each of the balloons contained a plastic disk with an internal catheter stem. A 20mm snare was used to capture this stem with successful removal in 3 of the cases, and in the other 2 cases a portion of the cut balloon was captured with the snare with resultant successful removal. The shortest procedure duration was 4 minutes with a thin balloon which was easily cut. The longest procedure duration was 45 minutes involving a thick balloon requiring increased effort for cutting. A 2T therapeutic double channel gastroscope was used in 3 cases (22-30 min), where a rat tooth forceps was used to hold the balloon while a pair of endoscopic scissors in the second channel cut the balloon hemi-circumferentially.

Figure: Case 1 (A-D) and Case 2 (E-H) of Endoscopic IGB removal.
Disclosures:
Murad Ali indicated no relevant financial relationships.
Rabia de Latour: Boston Scientific – Independent Contractor.
Murad H. Ali, MD1, Rabia de Latour, MD2. P3732 - Safe Endoscopic Removal of Unbranded, Internationally Placed Intra-Gastric Balloons Utilizing Regularly Available Endoscopic Tools, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
