Tuesday Poster Session
Category: Liver
P3788 - Baseline Characteristics and Risk Profiles of 1111 Patients with Primary Biliary Cholangitis (PBC) in Need of Second-Line Therapy
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
Has Audio

Gideon Hirschfield, PhD
Toronto Centre for Liver Disease
Toronto, ON, Canada
Presenting Author(s)
Gideon Hirschfield, PhD1, Kris Kowdley, MD, FACG2, Andreas Kremer, MD, PhD, MHBA3, John Vierling, MD4, Christopher Bowlus, MD5, Cynthia Levy, MD6, Marlyn Mayo, MD7, Daria Crittenden, MD8, Mary Standen, NP8, Ke Yang, PhD8, Yun-Jung Choi, PhD8, Charles McWherter, PhD8
1Toronto Centre for Liver Disease, Toronto, ON, Canada; 2Liver Institute Northwest, Seattle, WA; 3University of Zürich, Zurich, Zurich, Switzerland; 4Baylor College of Medicine, Houston, TX; 5UC Davis, Davis, CA; 6University of Miami Health System, Miami, FL; 7UT Southwestern, Dallas, TX; 8CymaBay Therapeutics, Newark, CA
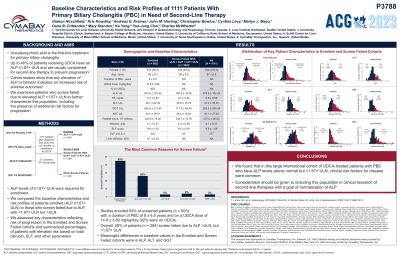
Introduction: Ursodexoxycholic acid (UDCA) is the first-line treatment for primary biliary cholangitis (PBC). Up to 40% of patients receiving UDCA have an alkaline phosphatase (ALP) ≥ 1.67×ULN and are usually considered for second-line therapy to prevent progression. Cohort studies confirm that targeting normal ALP and bilirubin are better treatment goals. We examined patients who screen failed due to elevated ALP < 1.67xULN to further characterize this population, including presence of additional risk factors for progression.
Methods: In four clinical trials with seladelpar from 2015 – 2022, 1111 patients with PBC were screened at 197 clinical sites in 27 countries after treatment with UDCA for ≥12 months, or intolerance to UDCA. ALP levels of ≥ 1.67×ULN were required for enrollment. We compared the baseline characteristics and risk profiles of patients enrolled (ALP ≥ 1.67×ULN) to those who screen failed due to ALP³ ULN, but < 1.67×ULN. We stratified using proportions with Enhanced Liver Fibrosis (ELF) values ≥ 10.0 or bilirubin levels > 0.6×ULN. The relationship of ELF and liver stiffness assessed with FibroScan® was confirmed when available.
Results: The 1111 screened patients were predominantly female (94%) with a mean (SD) age of 57±9.5 years. Studies enrolled 54% of screened patients (n=603) (EN cohort) with a duration of PBC of 8±6.5 years and on a UDCA dose of 15±3.9 mg/kg/day (92% were on UDCA). Overall, 26% of patients (n=284) screen failed due to ALP > ULN, but < 1.67×ULN (SF Cohort). The most common reasons for screen failure in the remaining 20% of patients were ALT and AST >3xULN, ALP< 1xULN, and EGFR < 60. Meaningful differences in baseline values in the EN and SF cohorts were in ALP, GGT and ALT. Higher-risk bilirubin levels were present in 51.1% and 42.0% of EN and SF cohorts, respectively. Elevated risk based on ELF was identified in 43.2% of the EN and 27.2% of the SF cohorts. Liver stiffness was assessed in 66% of EN patients, and a mean liver stiffness of 9.7 kPa correlated with ELF (r = 0.50, p < 0.001).
Discussion: We found that in this large international cohort of UDCA treated PBC patients, clinical risk factors for disease progression were common in patients achieving ALP levels > ULN, but < 1.67×ULN. Consideration should be given to including this population in clinical research of second-line therapies with a goal of normalization of ALP.

Disclosures:
Gideon Hirschfield, PhD1, Kris Kowdley, MD, FACG2, Andreas Kremer, MD, PhD, MHBA3, John Vierling, MD4, Christopher Bowlus, MD5, Cynthia Levy, MD6, Marlyn Mayo, MD7, Daria Crittenden, MD8, Mary Standen, NP8, Ke Yang, PhD8, Yun-Jung Choi, PhD8, Charles McWherter, PhD8. P3788 - Baseline Characteristics and Risk Profiles of 1111 Patients with Primary Biliary Cholangitis (PBC) in Need of Second-Line Therapy, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Toronto Centre for Liver Disease, Toronto, ON, Canada; 2Liver Institute Northwest, Seattle, WA; 3University of Zürich, Zurich, Zurich, Switzerland; 4Baylor College of Medicine, Houston, TX; 5UC Davis, Davis, CA; 6University of Miami Health System, Miami, FL; 7UT Southwestern, Dallas, TX; 8CymaBay Therapeutics, Newark, CA
Introduction: Ursodexoxycholic acid (UDCA) is the first-line treatment for primary biliary cholangitis (PBC). Up to 40% of patients receiving UDCA have an alkaline phosphatase (ALP) ≥ 1.67×ULN and are usually considered for second-line therapy to prevent progression. Cohort studies confirm that targeting normal ALP and bilirubin are better treatment goals. We examined patients who screen failed due to elevated ALP < 1.67xULN to further characterize this population, including presence of additional risk factors for progression.
Methods: In four clinical trials with seladelpar from 2015 – 2022, 1111 patients with PBC were screened at 197 clinical sites in 27 countries after treatment with UDCA for ≥12 months, or intolerance to UDCA. ALP levels of ≥ 1.67×ULN were required for enrollment. We compared the baseline characteristics and risk profiles of patients enrolled (ALP ≥ 1.67×ULN) to those who screen failed due to ALP³ ULN, but < 1.67×ULN. We stratified using proportions with Enhanced Liver Fibrosis (ELF) values ≥ 10.0 or bilirubin levels > 0.6×ULN. The relationship of ELF and liver stiffness assessed with FibroScan® was confirmed when available.
Results: The 1111 screened patients were predominantly female (94%) with a mean (SD) age of 57±9.5 years. Studies enrolled 54% of screened patients (n=603) (EN cohort) with a duration of PBC of 8±6.5 years and on a UDCA dose of 15±3.9 mg/kg/day (92% were on UDCA). Overall, 26% of patients (n=284) screen failed due to ALP > ULN, but < 1.67×ULN (SF Cohort). The most common reasons for screen failure in the remaining 20% of patients were ALT and AST >3xULN, ALP< 1xULN, and EGFR < 60. Meaningful differences in baseline values in the EN and SF cohorts were in ALP, GGT and ALT. Higher-risk bilirubin levels were present in 51.1% and 42.0% of EN and SF cohorts, respectively. Elevated risk based on ELF was identified in 43.2% of the EN and 27.2% of the SF cohorts. Liver stiffness was assessed in 66% of EN patients, and a mean liver stiffness of 9.7 kPa correlated with ELF (r = 0.50, p < 0.001).
Discussion: We found that in this large international cohort of UDCA treated PBC patients, clinical risk factors for disease progression were common in patients achieving ALP levels > ULN, but < 1.67×ULN. Consideration should be given to including this population in clinical research of second-line therapies with a goal of normalization of ALP.

Figure: Figure 1
Disclosures:
Gideon Hirschfield indicated no relevant financial relationships.
Kris Kowdley: 89Bio – Consultant, Grant/Research Support. Abbvie – Speakers Bureau. Callidatas – Consultant. CTI – Advisory Committee/Board Member. CymaBay – Consultant. Durect – Advisory Committee/Board Member. GENFIT – Consultant, Grant/Research Support. Gilead – Consultant, Grant/Research Support, Speakers Bureau. GSK – Grant/Research Support. Hanmi – Grant/Research Support. HighTide – Grant/Research Support. Inipharm – Consultant. Intercept – Consultant, Grant/Research Support, Speakers Bureau. Labcorp – Advisory Committee/Board Member. Madrigal – Consultant, Grant/Research Support. Mirum – Consultant, Grant/Research Support. NGM – Consultant, Grant/Research Support. Pfizer – Grant/Research Support. Pliant – Consultant, Grant/Research Support. Sonic Insight – receipt of equipment, materials, drugs, medical writing, gifts for other services. Viking – Grant/Research Support.
Andreas Kremer: AbbVie – Consultant. Bristol Myers Squibb – Consultant. CymaBay Therapeutics – Consultant. Eisai – Consultant. Escient – Consultant. Falk – Consultant. Gilead – Consultant. GSK – Consultant. Intercept Pharmaceuticals – Consultant, Grant/Research Support. Janssen – Consultant. Lilly – Consultant. Mirum – Consultant. MSD – Consultant. Vifor – Consultant. Zambon – Consultant.
John Vierling indicated no relevant financial relationships.
Christopher Bowlus indicated no relevant financial relationships.
Cynthia Levy indicated no relevant financial relationships.
Marlyn Mayo indicated no relevant financial relationships.
Daria Crittenden indicated no relevant financial relationships.
Mary Standen indicated no relevant financial relationships.
Ke Yang indicated no relevant financial relationships.
Yun-Jung Choi indicated no relevant financial relationships.
Charles McWherter indicated no relevant financial relationships.
Gideon Hirschfield, PhD1, Kris Kowdley, MD, FACG2, Andreas Kremer, MD, PhD, MHBA3, John Vierling, MD4, Christopher Bowlus, MD5, Cynthia Levy, MD6, Marlyn Mayo, MD7, Daria Crittenden, MD8, Mary Standen, NP8, Ke Yang, PhD8, Yun-Jung Choi, PhD8, Charles McWherter, PhD8. P3788 - Baseline Characteristics and Risk Profiles of 1111 Patients with Primary Biliary Cholangitis (PBC) in Need of Second-Line Therapy, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
