Tuesday Poster Session
Category: Esophagus
P3281 - Analysis of Reported Adverse Events Related to the Magnetic Sphincter Augmentation Reflux Management System: An FDA MAUDE Database Study
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
Has Audio

Khalid Ahmed, MD
The Wright Center for GME
Scranton, PA
Presenting Author(s)
Award: Presidential Poster Award
Khalid Ahmed, MD1, Manasik Abdu, MD2, Ayman Elawad, MD3, Abdellatif Ismail, MBBS, MD4, Peter Iskander, MD5, Mouhand FH. Mohamed, MD6, Fouad Jaber, MD7, Natalie Wilson, MD8, Osama Hamid, MD, MRCPI9, Khaled Elfert, MD10, Tessa Herman, MD8, Mohamed Abdallah, MD8, Mohammad Bilal, MD11
1The Wright Center for GME, Scranton, PA; 2University at Buffalo-Catholic Health System, Buffalo, NY; 3Howard University Hospital, Washington, DC; 4University of Maryland Medical Center Midtown Campus, Baltimore, MD; 5The Wright Center for GME, Dunmore, PA; 6Brown University, Providence, RI; 7University of Missouri-Kansas City, Kansas City, MO; 8University of Minnesota, Minneapolis, MN; 9Cleveland Clinic, Cleveland, OH; 10SBH Health System, Bronx, NY; 11Minneapolis VA Medical Center, Minneapolis, MN
Introduction: Surgical intervention is recommended when gastroesophageal reflux (GERD) is refractory to medical therapy. Magnetic sphincter augmentation (MSA) using the LINX® Reflux Management System (RMS) has been gaining popularity as a minimally invasive approach for management of GERD. However, there is limited data regarding the adverse events of the MSA RMS. Therefore, we aim to report and analyze adverse events associated with MSA RMS using the FDA's "Manufacturer and User Facility Device Experience" database.
Methods: We analyzed the post-marketing surveillance data from the FDA’s Manufacturer and User Facility Device Experience database for MSA RMS from December 2016 through April 2023. The results of the search were classified into patient-related adverse events and device-related technical issues. We also reported whether the device was explanted after the problem was identified.
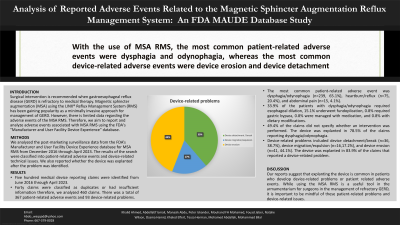
Results: Five hundred medical device reporting claims were identified from June 2016 through April 2023. Forty claims were classified as duplicates or had insufficient information therefore, we analyzed 460 claims. There was a total of 367 patient-related adverse events and 93 device-related problems. The most common patient-related adverse event was dysphagia/odynophagia (n=239, 65.1%), heartburn/reflux (n=75, 20.4%), and abdominal pain (n=15, 4.1%). 33.9% of the patients with dysphagia/odynophagia required esophageal dilation, 15.1% underwent fundoplication, 0.8% required gastric bypass, 0.8% were managed with medication, and 0.8% with dietary modifications. 49.4% of the claims did not specify whether an intervention was performed(Table 1). The device was explanted in 78.5% of the claims reporting dysphagia/odynophagia. Device-related problems included device detachment/break (n=36, 38.7%), device migration/expulsion (n=16, 17.2%), and device erosion (n=41, 44.1%)[Figure 1]. The device was explanted in 83.9% of the claims that reported a device-related problem.
Discussion: Our reports suggest that explanting the device is common in patients who develop device-related problems or patient related adverse events. While using the MSA RMS is a useful tool in the armamentarium for surgeons in the management of refractory GERD, it is important for surgeons to be mindful of these patient-related problems and device-related issues.

Disclosures:
Khalid Ahmed, MD1, Manasik Abdu, MD2, Ayman Elawad, MD3, Abdellatif Ismail, MBBS, MD4, Peter Iskander, MD5, Mouhand FH. Mohamed, MD6, Fouad Jaber, MD7, Natalie Wilson, MD8, Osama Hamid, MD, MRCPI9, Khaled Elfert, MD10, Tessa Herman, MD8, Mohamed Abdallah, MD8, Mohammad Bilal, MD11. P3281 - Analysis of Reported Adverse Events Related to the Magnetic Sphincter Augmentation Reflux Management System: An FDA MAUDE Database Study, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
Khalid Ahmed, MD1, Manasik Abdu, MD2, Ayman Elawad, MD3, Abdellatif Ismail, MBBS, MD4, Peter Iskander, MD5, Mouhand FH. Mohamed, MD6, Fouad Jaber, MD7, Natalie Wilson, MD8, Osama Hamid, MD, MRCPI9, Khaled Elfert, MD10, Tessa Herman, MD8, Mohamed Abdallah, MD8, Mohammad Bilal, MD11
1The Wright Center for GME, Scranton, PA; 2University at Buffalo-Catholic Health System, Buffalo, NY; 3Howard University Hospital, Washington, DC; 4University of Maryland Medical Center Midtown Campus, Baltimore, MD; 5The Wright Center for GME, Dunmore, PA; 6Brown University, Providence, RI; 7University of Missouri-Kansas City, Kansas City, MO; 8University of Minnesota, Minneapolis, MN; 9Cleveland Clinic, Cleveland, OH; 10SBH Health System, Bronx, NY; 11Minneapolis VA Medical Center, Minneapolis, MN
Introduction: Surgical intervention is recommended when gastroesophageal reflux (GERD) is refractory to medical therapy. Magnetic sphincter augmentation (MSA) using the LINX® Reflux Management System (RMS) has been gaining popularity as a minimally invasive approach for management of GERD. However, there is limited data regarding the adverse events of the MSA RMS. Therefore, we aim to report and analyze adverse events associated with MSA RMS using the FDA's "Manufacturer and User Facility Device Experience" database.
Methods: We analyzed the post-marketing surveillance data from the FDA’s Manufacturer and User Facility Device Experience database for MSA RMS from December 2016 through April 2023. The results of the search were classified into patient-related adverse events and device-related technical issues. We also reported whether the device was explanted after the problem was identified.
Results: Five hundred medical device reporting claims were identified from June 2016 through April 2023. Forty claims were classified as duplicates or had insufficient information therefore, we analyzed 460 claims. There was a total of 367 patient-related adverse events and 93 device-related problems. The most common patient-related adverse event was dysphagia/odynophagia (n=239, 65.1%), heartburn/reflux (n=75, 20.4%), and abdominal pain (n=15, 4.1%). 33.9% of the patients with dysphagia/odynophagia required esophageal dilation, 15.1% underwent fundoplication, 0.8% required gastric bypass, 0.8% were managed with medication, and 0.8% with dietary modifications. 49.4% of the claims did not specify whether an intervention was performed(Table 1). The device was explanted in 78.5% of the claims reporting dysphagia/odynophagia. Device-related problems included device detachment/break (n=36, 38.7%), device migration/expulsion (n=16, 17.2%), and device erosion (n=41, 44.1%)[Figure 1]. The device was explanted in 83.9% of the claims that reported a device-related problem.
Discussion: Our reports suggest that explanting the device is common in patients who develop device-related problems or patient related adverse events. While using the MSA RMS is a useful tool in the armamentarium for surgeons in the management of refractory GERD, it is important for surgeons to be mindful of these patient-related problems and device-related issues.

Figure: Figure 1. Device related problems
Disclosures:
Khalid Ahmed indicated no relevant financial relationships.
Manasik Abdu indicated no relevant financial relationships.
Ayman Elawad indicated no relevant financial relationships.
Abdellatif Ismail indicated no relevant financial relationships.
Peter Iskander indicated no relevant financial relationships.
Mouhand Mohamed indicated no relevant financial relationships.
Fouad Jaber indicated no relevant financial relationships.
Natalie Wilson indicated no relevant financial relationships.
Osama Hamid indicated no relevant financial relationships.
Khaled Elfert indicated no relevant financial relationships.
Tessa Herman indicated no relevant financial relationships.
Mohamed Abdallah indicated no relevant financial relationships.
Mohammad Bilal: Boston Scientific – Consultant.
Khalid Ahmed, MD1, Manasik Abdu, MD2, Ayman Elawad, MD3, Abdellatif Ismail, MBBS, MD4, Peter Iskander, MD5, Mouhand FH. Mohamed, MD6, Fouad Jaber, MD7, Natalie Wilson, MD8, Osama Hamid, MD, MRCPI9, Khaled Elfert, MD10, Tessa Herman, MD8, Mohamed Abdallah, MD8, Mohammad Bilal, MD11. P3281 - Analysis of Reported Adverse Events Related to the Magnetic Sphincter Augmentation Reflux Management System: An FDA MAUDE Database Study, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.

