Tuesday Poster Session
Category: Liver
P3848 - Safety of Nonselective Beta-Blockers in Decompensated Liver Cirrhosis and Their Role in Inducing Hepatorenal Syndrome: Should Their Use Be Restricted in This Setting?
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
Has Audio

Faris Qaqish, MD
Staten Island University Hospital
Staten Island, New York
Presenting Author(s)
Reem Dimachkie, MD, Faris Qaqish, MD, Rola Sasso, MD, Shabnam Dehgani, MD, Ahmad Abou Yassine, MD, Liliane Deeb, MD
Staten Island University Hospital, Staten Island, NY
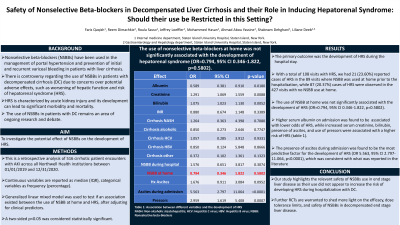
Introduction: Nonselective beta-blockers (NSBBs) have been used in the management of portal hypertension and prevention of initial and recurrent variceal bleeding in patients with liver cirrhosis. However, there is controversy regarding the use of NSBBs in patients with decompensated cirrhosis (DC) due to concerns over potential adverse effects, such as worsening of hepatic function and risk of hepatorenal syndrome (HRS). HRS is a serious complication of DC characterized by acute kidney injury (AKI) and progressive renal failure, and its development can lead to significant morbidity and mortality in this setting. Therefore, the use of NSBBs in patients with DC remains an area of ongoing research and debate. Our study aims to investigate the potential effect of NSBBs on the development of HRS.
Methods: This is a retrospective analysis of 516 cirrhotic patient encounters with AKI across all Northwell Health institutions between 01/01/2019 and 12/31/2020. Continuous variables are reported as median (IQR) and categorical variables as frequency (percentage). We used a generalized linear mixed model to test if an association existed between the use of NSBB at home and HRS, after adjusting for clinical predictors. A two-sided p< 0.05 was considered statistically significant. SAS 9.4 (SAS Institute Inc., Cary, NC) was used for statistical analysis.
Results: The primary outcome was the development of HRS during the hospital stay. With a total of 108 visits with HRS, we had 21(23.60%) reported HRS in the 89 visits where NSBB was used at home prior to the hospitalization, while 87(20.37%) HRS were observed in the 427 visits with no NSBB use at home. The use of NSBB at home was not significantly associated with the development of HRS (OR=0.794, 95% CI 0.346-1.822, p=0.5802). We also found that higher serum albumin on admission is associated with lower odds of HRS, while increased serum creatinine, bilirubin, presence of ascites, and use of pressors were associated with a higher risk of HRS (table below).
Discussion: Our study highlights the relevant safety of NSBBs use in end stage liver disease in regard to the fact that their use did not appear to increase the risk of developing HRS during hospitalization with decompensated cirrhosis. Further randomized controlled trials are warranted to shed more light on the efficacy, dose tolerance limits, and safety of NSBBs in decompensated end stage liver disease.
Disclosures:
Reem Dimachkie, MD, Faris Qaqish, MD, Rola Sasso, MD, Shabnam Dehgani, MD, Ahmad Abou Yassine, MD, Liliane Deeb, MD. P3848 - Safety of Nonselective Beta-Blockers in Decompensated Liver Cirrhosis and Their Role in Inducing Hepatorenal Syndrome: Should Their Use Be Restricted in This Setting?, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
Staten Island University Hospital, Staten Island, NY
Introduction: Nonselective beta-blockers (NSBBs) have been used in the management of portal hypertension and prevention of initial and recurrent variceal bleeding in patients with liver cirrhosis. However, there is controversy regarding the use of NSBBs in patients with decompensated cirrhosis (DC) due to concerns over potential adverse effects, such as worsening of hepatic function and risk of hepatorenal syndrome (HRS). HRS is a serious complication of DC characterized by acute kidney injury (AKI) and progressive renal failure, and its development can lead to significant morbidity and mortality in this setting. Therefore, the use of NSBBs in patients with DC remains an area of ongoing research and debate. Our study aims to investigate the potential effect of NSBBs on the development of HRS.
Methods: This is a retrospective analysis of 516 cirrhotic patient encounters with AKI across all Northwell Health institutions between 01/01/2019 and 12/31/2020. Continuous variables are reported as median (IQR) and categorical variables as frequency (percentage). We used a generalized linear mixed model to test if an association existed between the use of NSBB at home and HRS, after adjusting for clinical predictors. A two-sided p< 0.05 was considered statistically significant. SAS 9.4 (SAS Institute Inc., Cary, NC) was used for statistical analysis.
Results: The primary outcome was the development of HRS during the hospital stay. With a total of 108 visits with HRS, we had 21(23.60%) reported HRS in the 89 visits where NSBB was used at home prior to the hospitalization, while 87(20.37%) HRS were observed in the 427 visits with no NSBB use at home. The use of NSBB at home was not significantly associated with the development of HRS (OR=0.794, 95% CI 0.346-1.822, p=0.5802). We also found that higher serum albumin on admission is associated with lower odds of HRS, while increased serum creatinine, bilirubin, presence of ascites, and use of pressors were associated with a higher risk of HRS (table below).
Discussion: Our study highlights the relevant safety of NSBBs use in end stage liver disease in regard to the fact that their use did not appear to increase the risk of developing HRS during hospitalization with decompensated cirrhosis. Further randomized controlled trials are warranted to shed more light on the efficacy, dose tolerance limits, and safety of NSBBs in decompensated end stage liver disease.
Disclosures:
Reem Dimachkie indicated no relevant financial relationships.
Faris Qaqish indicated no relevant financial relationships.
Rola Sasso indicated no relevant financial relationships.
Shabnam Dehgani indicated no relevant financial relationships.
Ahmad Abou Yassine indicated no relevant financial relationships.
Liliane Deeb indicated no relevant financial relationships.
Reem Dimachkie, MD, Faris Qaqish, MD, Rola Sasso, MD, Shabnam Dehgani, MD, Ahmad Abou Yassine, MD, Liliane Deeb, MD. P3848 - Safety of Nonselective Beta-Blockers in Decompensated Liver Cirrhosis and Their Role in Inducing Hepatorenal Syndrome: Should Their Use Be Restricted in This Setting?, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.

