Tuesday Poster Session
Category: Liver
P3858 - Efficacy of Immune Checkpoint Inhibitors and Their Combinations in Previously Treated Advanced Hepatocellular Carcinoma: A Systematic Review of Clinical Trials
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
Has Audio
- WA
Wajeeha Aiman, MBBS
Saint Michael's Medical Center
Newark, New Jersey
Presenting Author(s)
Wajeeha Aiman, MBBS1, Muhammad Ashar Ali, MD2, Mukarram Jamat Ali, MD3, Mohammad Nabil Rayad, MD4, Noreen Mirza, MD4, Hamid Shaaban, MD1, Gunwant Guron, MD1, Michael Maroules, MD5
1Saint Michael's Medical Center, Newark, NJ; 2St. Mary's and St. Clare's Hospital, New York Medical College, Denville, NJ; 3Howard University Hospital, Washington, DC; 4Saint Michael's Medical Center, New York Medical College, Newark, NJ; 5St. Mary's St. Clare's Hospital, NYMC, Newark, NJ
Introduction: Hepatocellular carcinoma (HCC) is increasing worldwide and is often diagnosed at an advanced stage with no curative treatment and are unresectable. HCC frequently has immunosuppressed microenvironment and therefore, immune checkpoint inhibitors (ICI) can be effective in these tumors. In this systematic review, we will assess the efficacy of ICIs and its combinations with tyrosine kinase inhibitors (TKIs) in previously treated HCC.
Methods: Literature search was performed on PubMed and Embase with Mesh and Emtree terms, “Hepatocellular Carcinoma”, AND “ICI” from the inception of data till 2/30/23. We screened 3,741 articles and included 1 randomized clinical trial (RCT, N=413) and 10 nonrandomized clinical trials (nRCTs, N=1534) based on inclusion criteria. Prisma guidelines were followed conducting this systematic review.
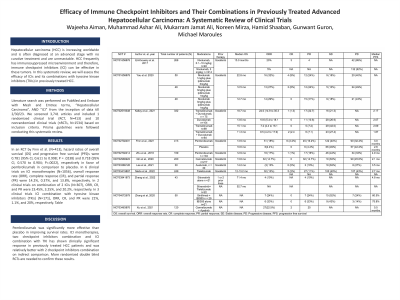
Results: In an RCT by Finn et al. (N=413), hazard ratios of overall survival (OS) and progression free survival (PFS)s were 0.781 (95% CI, 0.611 to 0.998; P = .0238) and 0.718 (95% CI, 0.570 to 0.904; P=.0022), respectively in favor of pembrolizumab in comparison to placebo. In 6 clinical trials on ICI monotherapies (N=1056), overall response rate (ORR), complete response (CR), and partial response (PR) were 14.2%, 0.37%, and 13.8%, respectively. In 2 clinical trials on combination of 2 ICIs (N=307), ORR, CR, and PR were 23.45%, 3.25%, and 20.2%, respectively. In 3 clinical trials ICI combination with tyrosine kinase inhibitors (TKIs) (N=171), ORR, CR, and PR were 21%, 1.1%, and 20%, respectively. Table
Discussion: Pembrolizumab was significantly more effective than placebo in improving survival rates. ICI monotherapies, two checkpoint inhibitors combination and ICI combination with TKI has shown clinically significant response in previously treated HCC patients and was relatively better with 2 checkpoint inhibitors combination on indirect comparison. More randomized double blind RCTs are needed to confirm these results.
Disclosures:
Wajeeha Aiman, MBBS1, Muhammad Ashar Ali, MD2, Mukarram Jamat Ali, MD3, Mohammad Nabil Rayad, MD4, Noreen Mirza, MD4, Hamid Shaaban, MD1, Gunwant Guron, MD1, Michael Maroules, MD5. P3858 - Efficacy of Immune Checkpoint Inhibitors and Their Combinations in Previously Treated Advanced Hepatocellular Carcinoma: A Systematic Review of Clinical Trials, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Saint Michael's Medical Center, Newark, NJ; 2St. Mary's and St. Clare's Hospital, New York Medical College, Denville, NJ; 3Howard University Hospital, Washington, DC; 4Saint Michael's Medical Center, New York Medical College, Newark, NJ; 5St. Mary's St. Clare's Hospital, NYMC, Newark, NJ
Introduction: Hepatocellular carcinoma (HCC) is increasing worldwide and is often diagnosed at an advanced stage with no curative treatment and are unresectable. HCC frequently has immunosuppressed microenvironment and therefore, immune checkpoint inhibitors (ICI) can be effective in these tumors. In this systematic review, we will assess the efficacy of ICIs and its combinations with tyrosine kinase inhibitors (TKIs) in previously treated HCC.
Methods: Literature search was performed on PubMed and Embase with Mesh and Emtree terms, “Hepatocellular Carcinoma”, AND “ICI” from the inception of data till 2/30/23. We screened 3,741 articles and included 1 randomized clinical trial (RCT, N=413) and 10 nonrandomized clinical trials (nRCTs, N=1534) based on inclusion criteria. Prisma guidelines were followed conducting this systematic review.
Results: In an RCT by Finn et al. (N=413), hazard ratios of overall survival (OS) and progression free survival (PFS)s were 0.781 (95% CI, 0.611 to 0.998; P = .0238) and 0.718 (95% CI, 0.570 to 0.904; P=.0022), respectively in favor of pembrolizumab in comparison to placebo. In 6 clinical trials on ICI monotherapies (N=1056), overall response rate (ORR), complete response (CR), and partial response (PR) were 14.2%, 0.37%, and 13.8%, respectively. In 2 clinical trials on combination of 2 ICIs (N=307), ORR, CR, and PR were 23.45%, 3.25%, and 20.2%, respectively. In 3 clinical trials ICI combination with tyrosine kinase inhibitors (TKIs) (N=171), ORR, CR, and PR were 21%, 1.1%, and 20%, respectively. Table
Discussion: Pembrolizumab was significantly more effective than placebo in improving survival rates. ICI monotherapies, two checkpoint inhibitors combination and ICI combination with TKI has shown clinically significant response in previously treated HCC patients and was relatively better with 2 checkpoint inhibitors combination on indirect comparison. More randomized double blind RCTs are needed to confirm these results.
Disclosures:
Wajeeha Aiman indicated no relevant financial relationships.
Muhammad Ashar Ali indicated no relevant financial relationships.
Mukarram Jamat Ali indicated no relevant financial relationships.
Mohammad Nabil Rayad indicated no relevant financial relationships.
Noreen Mirza indicated no relevant financial relationships.
Hamid Shaaban indicated no relevant financial relationships.
Gunwant Guron indicated no relevant financial relationships.
Michael Maroules indicated no relevant financial relationships.
Wajeeha Aiman, MBBS1, Muhammad Ashar Ali, MD2, Mukarram Jamat Ali, MD3, Mohammad Nabil Rayad, MD4, Noreen Mirza, MD4, Hamid Shaaban, MD1, Gunwant Guron, MD1, Michael Maroules, MD5. P3858 - Efficacy of Immune Checkpoint Inhibitors and Their Combinations in Previously Treated Advanced Hepatocellular Carcinoma: A Systematic Review of Clinical Trials, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
