Tuesday Poster Session
Category: Biliary/Pancreas
P2891 - Documented Patient Contact Increases Follow Up for Biliary Stent Removal Post ERCP at a Single Tertiary Care Safety Net Hospital: A Quality Improvement Project
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
Has Audio
- SV
Shyam Vedantam, DO
University of Miami/Jackson Memorial Hospital
Miami, FL
Presenting Author(s)
Shyam Vedantam, DO1, Rahil Shah, MD1, Jodie A. Barkin, MD, FACG2, Shria Kumar, MD, MSc(Epi)3, Sunil Amin, MD, MPH2, Sean Bhalla, MD2, Ami Panara Shukla, MD1
1University of Miami/Jackson Memorial Hospital, Miami, FL; 2University of Miami, Leonard M. Miller School of Medicine, Miami, FL; 3University of Miami, Miami, FL
Introduction: Biliary stents are placed during endoscopic retrograde cholangiopancreatography (ERCP) for obstruction and should be removed or exchanged in follow up to prevent complications. There is limited data regarding the barriers to follow up for stent removal. We analyzed the rate of follow up ERCP and potential contributing factors at a tertiary safety-net hospital.
Methods: This is a retrospective study of patients who underwent biliary stent placement during index ERCP from June 2017 to March 2019, with follow-up until June 2019. We excluded patients < 18 years old and those with bare metal stents placed for malignancy. We performed binary logistic regression on data including demographics, insurance type, transplant or non-transplant patients, setting of encounter, and documented patient contact.
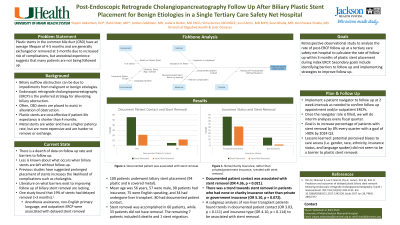
Results: 100 patients with biliary stent placement (94 plastic and 6 covered metal) were included. The mean age was 55.69 ± 15.03 years, with 43 (43%) female. 90 (90%) patients were insured. 75 (75%) patients considered English their primary language. 34 (34%) were transplant patients. 15 (15%) patients were uninsured or had charity insurance. Documented patient contact was achieved in 80 (80%) of patients. 60 (60%) patients had stents removed, 33 (33%) never had removal, and the remainder included migrated stents or deaths. Of the group that had stents removed, 34 stents (57%) were removed after the suggested removal date with an average time of 32.25 ± 43.68 days past recommended removal. Documented patient contact was the only variable significantly associated with stent removal (odds ratio [OR] 4.36, p = 0.021), although insurance type of none/charity, rather than private or government, also trended towards improved chances of stent removal (OR 5.10, p = 0.072). Subgroup analysis in the non-transplant patients showed that there were trends with documented patient contact (p = 0.111) and insurance type (p = 0.114) with successful stent removal, although neither was statistically significant in liver transplant patients.
Discussion: We demonstrate that 60% of stents were successfully removed and documented patient contact was associated with stent removal at a tertiary safety-net hospital. However, a large number of patients did not have their stents removed or presented after the recommended removal date. Based on this preliminary data, implementing a detailed patient contact protocol may address this gap in care, improve follow up, and enable successful stent removal.
Disclosures:
Shyam Vedantam, DO1, Rahil Shah, MD1, Jodie A. Barkin, MD, FACG2, Shria Kumar, MD, MSc(Epi)3, Sunil Amin, MD, MPH2, Sean Bhalla, MD2, Ami Panara Shukla, MD1. P2891 - Documented Patient Contact Increases Follow Up for Biliary Stent Removal Post ERCP at a Single Tertiary Care Safety Net Hospital: A Quality Improvement Project, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1University of Miami/Jackson Memorial Hospital, Miami, FL; 2University of Miami, Leonard M. Miller School of Medicine, Miami, FL; 3University of Miami, Miami, FL
Introduction: Biliary stents are placed during endoscopic retrograde cholangiopancreatography (ERCP) for obstruction and should be removed or exchanged in follow up to prevent complications. There is limited data regarding the barriers to follow up for stent removal. We analyzed the rate of follow up ERCP and potential contributing factors at a tertiary safety-net hospital.
Methods: This is a retrospective study of patients who underwent biliary stent placement during index ERCP from June 2017 to March 2019, with follow-up until June 2019. We excluded patients < 18 years old and those with bare metal stents placed for malignancy. We performed binary logistic regression on data including demographics, insurance type, transplant or non-transplant patients, setting of encounter, and documented patient contact.
Results: 100 patients with biliary stent placement (94 plastic and 6 covered metal) were included. The mean age was 55.69 ± 15.03 years, with 43 (43%) female. 90 (90%) patients were insured. 75 (75%) patients considered English their primary language. 34 (34%) were transplant patients. 15 (15%) patients were uninsured or had charity insurance. Documented patient contact was achieved in 80 (80%) of patients. 60 (60%) patients had stents removed, 33 (33%) never had removal, and the remainder included migrated stents or deaths. Of the group that had stents removed, 34 stents (57%) were removed after the suggested removal date with an average time of 32.25 ± 43.68 days past recommended removal. Documented patient contact was the only variable significantly associated with stent removal (odds ratio [OR] 4.36, p = 0.021), although insurance type of none/charity, rather than private or government, also trended towards improved chances of stent removal (OR 5.10, p = 0.072). Subgroup analysis in the non-transplant patients showed that there were trends with documented patient contact (p = 0.111) and insurance type (p = 0.114) with successful stent removal, although neither was statistically significant in liver transplant patients.
Discussion: We demonstrate that 60% of stents were successfully removed and documented patient contact was associated with stent removal at a tertiary safety-net hospital. However, a large number of patients did not have their stents removed or presented after the recommended removal date. Based on this preliminary data, implementing a detailed patient contact protocol may address this gap in care, improve follow up, and enable successful stent removal.
Disclosures:
Shyam Vedantam indicated no relevant financial relationships.
Rahil Shah indicated no relevant financial relationships.
Jodie Barkin: AbbVie – Advisor or Review Panel Member. Aimmune Therapeutics, a Nestlé Health Science company – Consultant. CorEvitas, LLC – Advisor or Review Panel Member. Envara Health – Advisor or Review Panel Member. Medtronic – Advisor or Review Panel Member. Organon – Advisor or Review Panel Member.
Shria Kumar indicated no relevant financial relationships.
Sunil Amin: Boston Scientific – Consultant. Medtronic – Consultant.
Sean Bhalla indicated no relevant financial relationships.
Ami Panara Shukla indicated no relevant financial relationships.
Shyam Vedantam, DO1, Rahil Shah, MD1, Jodie A. Barkin, MD, FACG2, Shria Kumar, MD, MSc(Epi)3, Sunil Amin, MD, MPH2, Sean Bhalla, MD2, Ami Panara Shukla, MD1. P2891 - Documented Patient Contact Increases Follow Up for Biliary Stent Removal Post ERCP at a Single Tertiary Care Safety Net Hospital: A Quality Improvement Project, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
