Tuesday Poster Session
Category: Liver
P3937 - From Herb to Harm: A Rare Encounter of Ashwagandha-Induced Liver Injury
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
Has Audio

Galvin Dhaliwal, MD
Southwest Healthcare MEC
RICHMOND, VA
Presenting Author(s)
Galvin Dhaliwal, MD1, Mueez Hussain, MD1, Ranjit Sivanandham, MBBS1, Jaspreet Dhillon, MD1, Sehar Hussain, 2, Indraneel Chakrabarty, MD, MA1
1Southwest Healthcare MEC, Temecula, CA; 2California University of Science and Medicine, Colton, CA
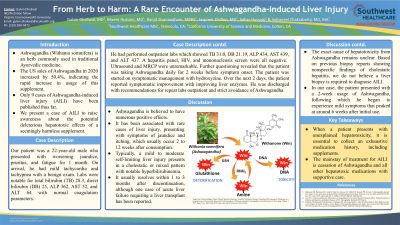
Introduction: Ashwagandha (Withania somnifera) is an herb commonly used in traditional Ayurvedic medicine. The US sales of Ashwagandha in 2020 increased by 50.4%, indicating the rapid increase in usage of this supplement. Only 9 cases of Ashwagandha-induced liver injury (AILI) have been published thus far. We present a case of AILI to raise awareness about the potential deleterious hepatotoxic effects of a seemingly harmless supplement.
Case Description/Methods: Our patient was a 22-year-old male who presented with worsening jaundice, pruritus, and fatigue for 1 month. On arrival, he had mild tachycardia and tachypnea with a benign exam. Labs were notable for total bilirubin (TB) 28.3, direct bilirubin (DB) 25, ALP 362, AST 52, and ALT 64 with normal coagulation parameters. He had performed outpatient labs which showed TB 31.0, DB 21.19, ALP 434, AST 439, and ALT 437. A hepatitis panel, HIV, and mononucleosis screen were all negative. Ultrasound and MRCP were unremarkable. Further questioning revealed that the patient was taking Ashwagandha daily for 2 weeks before symptom onset. The patient was started on symptomatic management with hydroxyzine. Over the next 2 days, the patient reported symptomatic improvement with improving liver enzymes. He was discharged with recommendations for repeat labs outpatient and strict avoidance of Ashwagandha.
Discussion: Ashwagandha is believed to have numerous positive effects. It has been associated with rare cases of liver injury, presenting with symptoms of jaundice and itching, which usually occur 2 to 12 weeks after consumption. Typically, a mild to moderate self-limiting liver injury presents in a cholestatic or mixed pattern with notable hyperbilirubinemia. It usually resolves within 1 to 3 months after discontinuation, although one case of acute liver failure requiring a liver transplant has been reported. The exact cause of hepatotoxicity from Ashwagandha remains unclear. Based on previous biopsy reports showing nonspecific findings of cholestatic hepatitis, we do not believe a liver biopsy is required to diagnose AILI. In our case, the patient presented with a 2-week usage of Ashwagandha, following which he began to experience mild symptoms that peaked at around 6 weeks after initial use. When a patient presents with unexplained hepatotoxicity, it is essential to collect an exhaustive medication history, including supplements. The mainstay of treatment for AILI is cessation of Ashwagandha and all other hepatotoxic medications with supportive care.
Disclosures:
Galvin Dhaliwal, MD1, Mueez Hussain, MD1, Ranjit Sivanandham, MBBS1, Jaspreet Dhillon, MD1, Sehar Hussain, 2, Indraneel Chakrabarty, MD, MA1. P3937 - From Herb to Harm: A Rare Encounter of Ashwagandha-Induced Liver Injury, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Southwest Healthcare MEC, Temecula, CA; 2California University of Science and Medicine, Colton, CA
Introduction: Ashwagandha (Withania somnifera) is an herb commonly used in traditional Ayurvedic medicine. The US sales of Ashwagandha in 2020 increased by 50.4%, indicating the rapid increase in usage of this supplement. Only 9 cases of Ashwagandha-induced liver injury (AILI) have been published thus far. We present a case of AILI to raise awareness about the potential deleterious hepatotoxic effects of a seemingly harmless supplement.
Case Description/Methods: Our patient was a 22-year-old male who presented with worsening jaundice, pruritus, and fatigue for 1 month. On arrival, he had mild tachycardia and tachypnea with a benign exam. Labs were notable for total bilirubin (TB) 28.3, direct bilirubin (DB) 25, ALP 362, AST 52, and ALT 64 with normal coagulation parameters. He had performed outpatient labs which showed TB 31.0, DB 21.19, ALP 434, AST 439, and ALT 437. A hepatitis panel, HIV, and mononucleosis screen were all negative. Ultrasound and MRCP were unremarkable. Further questioning revealed that the patient was taking Ashwagandha daily for 2 weeks before symptom onset. The patient was started on symptomatic management with hydroxyzine. Over the next 2 days, the patient reported symptomatic improvement with improving liver enzymes. He was discharged with recommendations for repeat labs outpatient and strict avoidance of Ashwagandha.
Discussion: Ashwagandha is believed to have numerous positive effects. It has been associated with rare cases of liver injury, presenting with symptoms of jaundice and itching, which usually occur 2 to 12 weeks after consumption. Typically, a mild to moderate self-limiting liver injury presents in a cholestatic or mixed pattern with notable hyperbilirubinemia. It usually resolves within 1 to 3 months after discontinuation, although one case of acute liver failure requiring a liver transplant has been reported. The exact cause of hepatotoxicity from Ashwagandha remains unclear. Based on previous biopsy reports showing nonspecific findings of cholestatic hepatitis, we do not believe a liver biopsy is required to diagnose AILI. In our case, the patient presented with a 2-week usage of Ashwagandha, following which he began to experience mild symptoms that peaked at around 6 weeks after initial use. When a patient presents with unexplained hepatotoxicity, it is essential to collect an exhaustive medication history, including supplements. The mainstay of treatment for AILI is cessation of Ashwagandha and all other hepatotoxic medications with supportive care.
Disclosures:
Galvin Dhaliwal indicated no relevant financial relationships.
Mueez Hussain indicated no relevant financial relationships.
Ranjit Sivanandham indicated no relevant financial relationships.
Jaspreet Dhillon indicated no relevant financial relationships.
Sehar Hussain indicated no relevant financial relationships.
Indraneel Chakrabarty indicated no relevant financial relationships.
Galvin Dhaliwal, MD1, Mueez Hussain, MD1, Ranjit Sivanandham, MBBS1, Jaspreet Dhillon, MD1, Sehar Hussain, 2, Indraneel Chakrabarty, MD, MA1. P3937 - From Herb to Harm: A Rare Encounter of Ashwagandha-Induced Liver Injury, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
