Tuesday Poster Session
Category: Liver
P3962 - Nodular Regenerative Hyperplasia in a Patient Treated for Triple Positive Breast Cancer
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
Has Audio

Tyrell Daniel, MD
Allegheny General Hospital
Pittsburgh, PA
Presenting Author(s)
Tyrell Daniel, MD1, Sheena Mago, DO2, Palgun Nisarga, MD2, Mark Bunker, MD2, Helen Analo, MD2
1Allegheny General Hospital, Pittsburgh, PA; 2Allegheny Health Network, Pittsburgh, PA
Introduction: Nodular regenerative hyperplasia (NRH) of the liver is a rare disease caused by the benign hyperproliferation of hepatocytes caused by obstructive portal venopathy and the resulting abnormal perfusion. Etiologies include autoimmune, infectious or drug related causes. NRH may progress to complications of non-cirrhotic portal hypertension (NCPH). Here we present a case of a woman with triple positive breast cancer treated with Ado-trastuzumab emtansine (Kadcyla) who developed NRH.
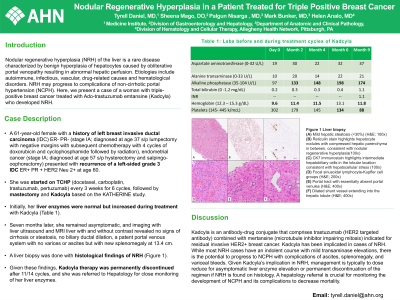
Case Description/Methods: A 61-year-old female with a history of left breast invasive ductal carcinoma (IDC) ER- PR- (stage IA; diagnosed at age 37 status post lumpectomy with negative margins with subsequent chemotherapy with 4 cycles of doxorubicin and cyclophosphamide followed by radiation), endometrial cancer (stage IA; diagnosed at age 57 status post hysterectomy and salpingo-oophorectomy) presented with recurrence of a left breast mass at age 60 found to be grade 3 IDC ER+ PR + HER2 Neu 2+. She was started on TCHP (docetaxel, carboplatin, trastuzumab, pertuzumab) every 3 weeks for 6 cycles followed by mastectomy and then Kadcyla based on the KATHERINE study. Initially, her liver enzymes were normal but increased during treatment with Kadcyla (Table 1). Seven months later, she remained asymptomatic and imaging with liver ultrasound and MRI liver with and without contrast revealed no signs of cirrhosis or steatosis, no biliary ductal dilation, a patent portal venous system with no varices or ascites but with new splenomegaly at 13.4 cm. A liver biopsy was done with histological findings of NRH (Figure 1). Given these findings, Kadcyla therapy was permanently discontinued after 11/14 cycles and she was referred to Hepatology for close monitoring of her liver enzymes.
Discussion: Kadcyla is an antibody-drug conjugate that compromises trastuzumab (HER2 targeted antibody) combined with mertansine (microtubule inhibitor impairing mitosis) indicated for residual invasive HER2+ breast cancer. Kadcyla has been implicated in cases of NRH. While most NRH cases have an indolent course with mild transaminase elevations, there is the potential to progress to NCPH with complications of ascites, splenomegaly, variceal bleeds. Given Kadcyla’s implication in NRH, management is typically to dose reduce for asymptomatic liver enzyme elevation or permanent discontinuation of the regimen if NRH is found on histology. A hepatology referral is crucial for monitoring for the development on NCPH and its complications to decrease mortality.

Disclosures:
Tyrell Daniel, MD1, Sheena Mago, DO2, Palgun Nisarga, MD2, Mark Bunker, MD2, Helen Analo, MD2. P3962 - Nodular Regenerative Hyperplasia in a Patient Treated for Triple Positive Breast Cancer, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Allegheny General Hospital, Pittsburgh, PA; 2Allegheny Health Network, Pittsburgh, PA
Introduction: Nodular regenerative hyperplasia (NRH) of the liver is a rare disease caused by the benign hyperproliferation of hepatocytes caused by obstructive portal venopathy and the resulting abnormal perfusion. Etiologies include autoimmune, infectious or drug related causes. NRH may progress to complications of non-cirrhotic portal hypertension (NCPH). Here we present a case of a woman with triple positive breast cancer treated with Ado-trastuzumab emtansine (Kadcyla) who developed NRH.
Case Description/Methods: A 61-year-old female with a history of left breast invasive ductal carcinoma (IDC) ER- PR- (stage IA; diagnosed at age 37 status post lumpectomy with negative margins with subsequent chemotherapy with 4 cycles of doxorubicin and cyclophosphamide followed by radiation), endometrial cancer (stage IA; diagnosed at age 57 status post hysterectomy and salpingo-oophorectomy) presented with recurrence of a left breast mass at age 60 found to be grade 3 IDC ER+ PR + HER2 Neu 2+. She was started on TCHP (docetaxel, carboplatin, trastuzumab, pertuzumab) every 3 weeks for 6 cycles followed by mastectomy and then Kadcyla based on the KATHERINE study. Initially, her liver enzymes were normal but increased during treatment with Kadcyla (Table 1). Seven months later, she remained asymptomatic and imaging with liver ultrasound and MRI liver with and without contrast revealed no signs of cirrhosis or steatosis, no biliary ductal dilation, a patent portal venous system with no varices or ascites but with new splenomegaly at 13.4 cm. A liver biopsy was done with histological findings of NRH (Figure 1). Given these findings, Kadcyla therapy was permanently discontinued after 11/14 cycles and she was referred to Hepatology for close monitoring of her liver enzymes.
Discussion: Kadcyla is an antibody-drug conjugate that compromises trastuzumab (HER2 targeted antibody) combined with mertansine (microtubule inhibitor impairing mitosis) indicated for residual invasive HER2+ breast cancer. Kadcyla has been implicated in cases of NRH. While most NRH cases have an indolent course with mild transaminase elevations, there is the potential to progress to NCPH with complications of ascites, splenomegaly, variceal bleeds. Given Kadcyla’s implication in NRH, management is typically to dose reduce for asymptomatic liver enzyme elevation or permanent discontinuation of the regimen if NRH is found on histology. A hepatology referral is crucial for monitoring for the development on NCPH and its complications to decrease mortality.

Figure: Figure 1 Liver biopsy. (A) Mild hepatic steatosis (<30%).(H&E; 100x) (B) Reticulin stain highlights the varying zones hepatocyte thickness consistent with nodular regenerative hyperplasia. (100x) (C) CK7 immunostain highlights intermediate hepatobiliary cells in the lobular location consistent with hepatocellular stress. (100x) (D) Focal sinusoidal lymphocyte-Kupffer cell groups. (H&E; 200x) (E) Portal tract with essentially absent portal venules. (H&E; 400x) (F) Dilated shunt vessel extending into the hepatic lobule. (H&E; 400x)
Disclosures:
Tyrell Daniel indicated no relevant financial relationships.
Sheena Mago indicated no relevant financial relationships.
Palgun Nisarga indicated no relevant financial relationships.
Mark Bunker indicated no relevant financial relationships.
Helen Analo indicated no relevant financial relationships.
Tyrell Daniel, MD1, Sheena Mago, DO2, Palgun Nisarga, MD2, Mark Bunker, MD2, Helen Analo, MD2. P3962 - Nodular Regenerative Hyperplasia in a Patient Treated for Triple Positive Breast Cancer, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
