Tuesday Poster Session
Category: Liver
P3964 - Cholestatic Drug-Induced Liver Injury in a Patient Taking High-Dose Niacin for Hyperlipidemia
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
Has Audio

Nanjiba Nawaz, MD
Mayo Clinic Florida
Jacksonville, FL
Presenting Author(s)
Nanjiba Nawaz, MD1, Christian Karime, MD2, Tyler Mistretta, MD1, Emily Butts, MD1, Jason Lewis, MD1
1Mayo Clinic Florida, Jacksonville, FL; 2Mayo Clinic, Jacksonville, FL
Introduction: Niacin is an important component of a balanced diet. Occasionally used to treat hyperlipidemia, niacin is widely available without prescription. Severe hepatotoxicity with niacin use has been reported. We describe a case of acute decompensated cirrhosis in a patient taking self-initiated high-dose niacin.
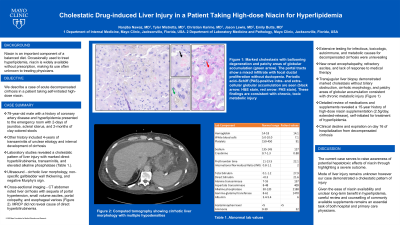
Case Description/Methods: A 79-year-old male with a history of coronary artery disease and hyperlipidemia presented to the emergency room with two days of jaundice and scleral icterus as well as two-month history of clay-colored stools. Questioning revealed a four-year history of transaminitis of unclear etiology and interval development of cirrhosis. Laboratory studies were consistent with cholestatic pattern of liver injury with marked direct hyperbilirubinemia, transaminitis, and elevated alkaline phosphatase. Calculated MELD-Na score was 30. Sonography noted cirrhotic liver morphology, non-specific gallbladder wall thickening, and negative Murphy’s sign. No biliary duct dilatation, obstruction, or biliary sludge was noted. Further imaging with magnetic resonance cholangiopancreatography did not reveal cause of direct hyperbilirubinemia. Extensive investigation of infectious, toxicologic, autoimmune, and metabolic causes for decompensated cirrhosis were unrevealing. Transjugular liver biopsy demonstrated marked cholestasis without biliary obstruction, cirrhotic morphology, and patchy areas of globular accumulation consistent with chronic metabolic injury (Figure 1). Detailed review of medications and supplements revealed a 15-year history of high-dose niacin supplementation (2.5g/day, extended-release), self-initiated for treatment of hyperlipidemia. Despite escalating medical management, the patient continued to clinically decline and expired on day 16 of hospitalization.
Discussion: Given the ease of niacin availability and unclear long-term benefit in hyperlipidemia, the current case serves to raise awareness of potential hepatotoxic effects through highlighting a severe outcome. Although mode of liver injury remains unknown, use of extended-release niacin formulations and prolonged high-dose supplementation is associated with enhanced hepatotoxicity. Careful review and counselling of commonly available supplements remains an important task of both hospital and primary care physicians.

Disclosures:
Nanjiba Nawaz, MD1, Christian Karime, MD2, Tyler Mistretta, MD1, Emily Butts, MD1, Jason Lewis, MD1. P3964 - Cholestatic Drug-Induced Liver Injury in a Patient Taking High-Dose Niacin for Hyperlipidemia, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Mayo Clinic Florida, Jacksonville, FL; 2Mayo Clinic, Jacksonville, FL
Introduction: Niacin is an important component of a balanced diet. Occasionally used to treat hyperlipidemia, niacin is widely available without prescription. Severe hepatotoxicity with niacin use has been reported. We describe a case of acute decompensated cirrhosis in a patient taking self-initiated high-dose niacin.
Case Description/Methods: A 79-year-old male with a history of coronary artery disease and hyperlipidemia presented to the emergency room with two days of jaundice and scleral icterus as well as two-month history of clay-colored stools. Questioning revealed a four-year history of transaminitis of unclear etiology and interval development of cirrhosis. Laboratory studies were consistent with cholestatic pattern of liver injury with marked direct hyperbilirubinemia, transaminitis, and elevated alkaline phosphatase. Calculated MELD-Na score was 30. Sonography noted cirrhotic liver morphology, non-specific gallbladder wall thickening, and negative Murphy’s sign. No biliary duct dilatation, obstruction, or biliary sludge was noted. Further imaging with magnetic resonance cholangiopancreatography did not reveal cause of direct hyperbilirubinemia. Extensive investigation of infectious, toxicologic, autoimmune, and metabolic causes for decompensated cirrhosis were unrevealing. Transjugular liver biopsy demonstrated marked cholestasis without biliary obstruction, cirrhotic morphology, and patchy areas of globular accumulation consistent with chronic metabolic injury (Figure 1). Detailed review of medications and supplements revealed a 15-year history of high-dose niacin supplementation (2.5g/day, extended-release), self-initiated for treatment of hyperlipidemia. Despite escalating medical management, the patient continued to clinically decline and expired on day 16 of hospitalization.
Discussion: Given the ease of niacin availability and unclear long-term benefit in hyperlipidemia, the current case serves to raise awareness of potential hepatotoxic effects through highlighting a severe outcome. Although mode of liver injury remains unknown, use of extended-release niacin formulations and prolonged high-dose supplementation is associated with enhanced hepatotoxicity. Careful review and counselling of commonly available supplements remains an important task of both hospital and primary care physicians.

Figure: Figure 1: Marked cholestasis with ballooning degeneration and patchy areas of globular accumulation (green arrow). The portal tracts show a mixed infiltrate with focal ductal proliferation without ductopenia. Periodic acid–Schiff (PAS)-positive intra- and extra-cellular globular accumulation are seen (black arrow: hematoxylin & eosin stain, red arrow: PAS stain). Genetic testing for Alpha-1-Antitrypsin is negative (normal phenotype - MM).
Disclosures:
Nanjiba Nawaz indicated no relevant financial relationships.
Christian Karime indicated no relevant financial relationships.
Tyler Mistretta indicated no relevant financial relationships.
Emily Butts indicated no relevant financial relationships.
Jason Lewis indicated no relevant financial relationships.
Nanjiba Nawaz, MD1, Christian Karime, MD2, Tyler Mistretta, MD1, Emily Butts, MD1, Jason Lewis, MD1. P3964 - Cholestatic Drug-Induced Liver Injury in a Patient Taking High-Dose Niacin for Hyperlipidemia, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.

