Tuesday Poster Session
Category: Liver
P3973 - Budesonide Can Successfully Treat Bosutanib-Induced Autoimmune Hepatitis
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
Has Audio

Jonathan L. Steinberg, MD
Stony Brook University Hospital
Stony Brook, NY
Presenting Author(s)
Michael Jorgensen, MD1, Jonathan L. Steinberg, MD1, Daniel S. Jamorabo, MD2
1Stony Brook University Hospital, Stony Brook, NY; 2Stony Brook Medicine, Stony Brook, NY
Introduction: Bosutinib, along with other tyrosine kinase inhibitors (TKI) used to treat Chronic Myelogenous Leukemia (CML), frequently causes a mild drug-induced liver injury (DILI), but no cases of drug-induced autoimmune hepatitis (DI-AIH) have been documented. We report a case of a woman on bosutinib for CML who was diagnosed with DI-AIH and was successfully treated with budesonide monotherapy.
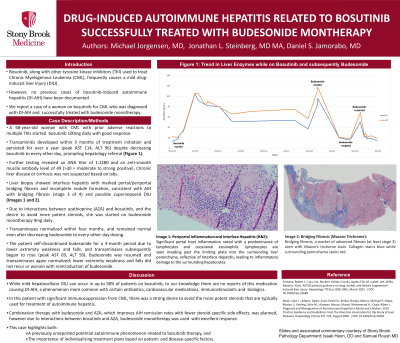
Case Description/Methods: A 68-year-old woman with CML who had developed adverse reactions to three different TKIs was started on bosutinib 100mg daily with good response. Within 3 months her transaminases began to rise, and despite decreasing the bosutinib to every other day they remained consistently elevated for the next year, with peak AST of 106 IU/L. She was thus referred to hepatology clinic.
Extensive liver work up revealed an ANA titer of 1:1280 and an anti-smooth muscle antibody level of 49 ( >30 = moderate to strong positive). There were no stigmata of chronic liver disease or cirrhosis. Subsequent liver biopsy showed interface hepatitis with marked portal/periportal bridging fibrosis and incomplete nodule formation, consistent with a diagnosis of AIH with bridging fibrosis (stage 3 of 4) and possible superimposed DILI.
Due to interactions between azathioprine (AZA) and bosutinib, and the desire to avoid more potent steroids, she was started on budesonide monotherapy 9mg daily; transaminases normalized within 4 months, and remained normal even after decreasing to every-other-day dosing because of loose stools. The patient later held budesonide for a 3-month period due to lower extremity weakness and falls, and her transaminases rose with peak AST 118 IU/L. Budesonide every other day was resumed and her transaminases again normalized with no further side effects.
Discussion: While mild hepatocellular DILI can occur in up to 58% of patients on bosutinib, to our knowledge there are no reports of this medication causing DI-AIH. In this patient with significant immunosuppression from CML, there was a strong desire to avoid more potent steroids. Combination therapy with budesonide and AZA, which improves AIH remission rates with fewer steroid-specific side effects, was planned, however due to potential interactions between bosutinib and AZA, she was treated with budesonide monotherapy with excellent response. This case highlights both a rare drug-induced autoimmune phenomenon, and the importance of individualizing treatment plans based on patient-specific factors.

Disclosures:
Michael Jorgensen, MD1, Jonathan L. Steinberg, MD1, Daniel S. Jamorabo, MD2. P3973 - Budesonide Can Successfully Treat Bosutanib-Induced Autoimmune Hepatitis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Stony Brook University Hospital, Stony Brook, NY; 2Stony Brook Medicine, Stony Brook, NY
Introduction: Bosutinib, along with other tyrosine kinase inhibitors (TKI) used to treat Chronic Myelogenous Leukemia (CML), frequently causes a mild drug-induced liver injury (DILI), but no cases of drug-induced autoimmune hepatitis (DI-AIH) have been documented. We report a case of a woman on bosutinib for CML who was diagnosed with DI-AIH and was successfully treated with budesonide monotherapy.
Case Description/Methods: A 68-year-old woman with CML who had developed adverse reactions to three different TKIs was started on bosutinib 100mg daily with good response. Within 3 months her transaminases began to rise, and despite decreasing the bosutinib to every other day they remained consistently elevated for the next year, with peak AST of 106 IU/L. She was thus referred to hepatology clinic.
Extensive liver work up revealed an ANA titer of 1:1280 and an anti-smooth muscle antibody level of 49 ( >30 = moderate to strong positive). There were no stigmata of chronic liver disease or cirrhosis. Subsequent liver biopsy showed interface hepatitis with marked portal/periportal bridging fibrosis and incomplete nodule formation, consistent with a diagnosis of AIH with bridging fibrosis (stage 3 of 4) and possible superimposed DILI.
Due to interactions between azathioprine (AZA) and bosutinib, and the desire to avoid more potent steroids, she was started on budesonide monotherapy 9mg daily; transaminases normalized within 4 months, and remained normal even after decreasing to every-other-day dosing because of loose stools. The patient later held budesonide for a 3-month period due to lower extremity weakness and falls, and her transaminases rose with peak AST 118 IU/L. Budesonide every other day was resumed and her transaminases again normalized with no further side effects.
Discussion: While mild hepatocellular DILI can occur in up to 58% of patients on bosutinib, to our knowledge there are no reports of this medication causing DI-AIH. In this patient with significant immunosuppression from CML, there was a strong desire to avoid more potent steroids. Combination therapy with budesonide and AZA, which improves AIH remission rates with fewer steroid-specific side effects, was planned, however due to potential interactions between bosutinib and AZA, she was treated with budesonide monotherapy with excellent response. This case highlights both a rare drug-induced autoimmune phenomenon, and the importance of individualizing treatment plans based on patient-specific factors.

Figure: Figure 1: Trend in Liver Enzymes with Treatment
Disclosures:
Michael Jorgensen indicated no relevant financial relationships.
Jonathan Steinberg indicated no relevant financial relationships.
Daniel Jamorabo indicated no relevant financial relationships.
Michael Jorgensen, MD1, Jonathan L. Steinberg, MD1, Daniel S. Jamorabo, MD2. P3973 - Budesonide Can Successfully Treat Bosutanib-Induced Autoimmune Hepatitis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
