Tuesday Poster Session
Category: Liver
P4017 - A Rare Case of Checkpoint Inhibitor Mediated Hepatitis in a Patient With Melanoma
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
Has Audio

Ramnik Gill, MD, MS
New York Presbyterian Brooklyn Methodist Hospital
Brooklyn, NY
Presenting Author(s)
Ramnik K. Gill, MD, MS1, Robinderpal Sandhu, MD2, Ezana Bekele, MD1, Meghaben Kothari, MD1
1New York Presbyterian Brooklyn Methodist, Brooklyn, NY; 2Mount Sinai Morningside-West, New York, NY
Introduction: With the rise of immune checkpoint inhibitors in the treatment regimen of multiple malignancies, many lesser-known complications are unveiled, such as checkpoint inhibitor mediated hepatitis (3% incidence). Our case highlights the presentation, diagnosis and treatment of a patient with Nivolumab induced hepatitis.
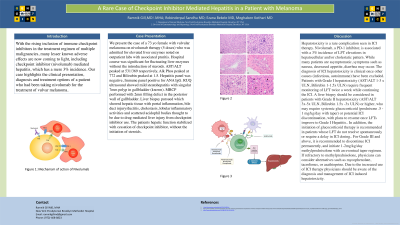
Case Description/Methods: We present the case of a female with vulvar melanoma on nivolumab therapy (5 doses), admitted for elevated liver enzymes on outpatient labs. Hospital course was significant for fluctuating liver enzymes without the introduction of steroids. AST/ALT peaked at 531/369 respectively, Alk Phos peaked at 772 and Bilirubin peaked at 1.5. Hepatitis panel was negative, Immune panel positive for ANA IgG. RUQ US showed mild steatohepatitis. MRCP showed 2mm filling defect in the posterior wall of the gallbladder. Liver biopsy showed portal inflammation, bile duct injury/ductitis, lobular inflammation and scattered acidophil bodies thought to be due to drug mediated liver injury from checkpoint inhibitors. The patients hepatic function stabilized with cessation of checkpoint inhibitor, without the initiation of steroids.
Discussion: Hepatotoxicity is a rare complication seen in ICI therapy. Nivolumab, a PD-1 inhibitor, is associated with a 3% incidence of LFT elevations in hepatocellular and/or cholestatic pattern. While many are asymptomatic, symptoms of nausea, cramping and diarrhea may occur. The diagnosis of ICI hepatotoxicity is clinical once other causes (infectious, autoimmune) have been excluded. Patients with Grade I hepatotoxicity (AST/ALT 1-3 x ULN, Bilirubin 1-1.5x ULN) require frequent monitoring of LFT while continuing the ICI. A liver biopsy can be considered in patients with Grade II hepatotoxicity (AST/ALT 3x-5x ULN, Bilirubin 1.5x -3x ULN) or higher, who may require systemic glucocorticoid (prednisone .5 - 1 mg/kg/day with taper) or potential ICI discontinuation, with plans to resume once LFTs improve to Grade I Hepatitis. In addition, the initiation of glucocorticoid therapy is recommended in patients whose LFT do not resolve spontaneously or require a delay in ICI dosing. For Grade III and above, it is recommended to discontinue ICI and initiate 1-2mg/kg/day methylprednisolone with an eventual taper. If refractory to methylprednisolone, physicians can consider alternatives such as mycophenolate, tacrolimus, or azathioprine. Due to the increased use of ICI therapy, physicians should be aware of the diagnosis and management of ICI induced hepatotoxicity.
Disclosures:
Ramnik K. Gill, MD, MS1, Robinderpal Sandhu, MD2, Ezana Bekele, MD1, Meghaben Kothari, MD1. P4017 - A Rare Case of Checkpoint Inhibitor Mediated Hepatitis in a Patient With Melanoma, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1New York Presbyterian Brooklyn Methodist, Brooklyn, NY; 2Mount Sinai Morningside-West, New York, NY
Introduction: With the rise of immune checkpoint inhibitors in the treatment regimen of multiple malignancies, many lesser-known complications are unveiled, such as checkpoint inhibitor mediated hepatitis (3% incidence). Our case highlights the presentation, diagnosis and treatment of a patient with Nivolumab induced hepatitis.
Case Description/Methods: We present the case of a female with vulvar melanoma on nivolumab therapy (5 doses), admitted for elevated liver enzymes on outpatient labs. Hospital course was significant for fluctuating liver enzymes without the introduction of steroids. AST/ALT peaked at 531/369 respectively, Alk Phos peaked at 772 and Bilirubin peaked at 1.5. Hepatitis panel was negative, Immune panel positive for ANA IgG. RUQ US showed mild steatohepatitis. MRCP showed 2mm filling defect in the posterior wall of the gallbladder. Liver biopsy showed portal inflammation, bile duct injury/ductitis, lobular inflammation and scattered acidophil bodies thought to be due to drug mediated liver injury from checkpoint inhibitors. The patients hepatic function stabilized with cessation of checkpoint inhibitor, without the initiation of steroids.
Discussion: Hepatotoxicity is a rare complication seen in ICI therapy. Nivolumab, a PD-1 inhibitor, is associated with a 3% incidence of LFT elevations in hepatocellular and/or cholestatic pattern. While many are asymptomatic, symptoms of nausea, cramping and diarrhea may occur. The diagnosis of ICI hepatotoxicity is clinical once other causes (infectious, autoimmune) have been excluded. Patients with Grade I hepatotoxicity (AST/ALT 1-3 x ULN, Bilirubin 1-1.5x ULN) require frequent monitoring of LFT while continuing the ICI. A liver biopsy can be considered in patients with Grade II hepatotoxicity (AST/ALT 3x-5x ULN, Bilirubin 1.5x -3x ULN) or higher, who may require systemic glucocorticoid (prednisone .5 - 1 mg/kg/day with taper) or potential ICI discontinuation, with plans to resume once LFTs improve to Grade I Hepatitis. In addition, the initiation of glucocorticoid therapy is recommended in patients whose LFT do not resolve spontaneously or require a delay in ICI dosing. For Grade III and above, it is recommended to discontinue ICI and initiate 1-2mg/kg/day methylprednisolone with an eventual taper. If refractory to methylprednisolone, physicians can consider alternatives such as mycophenolate, tacrolimus, or azathioprine. Due to the increased use of ICI therapy, physicians should be aware of the diagnosis and management of ICI induced hepatotoxicity.
Disclosures:
Ramnik Gill indicated no relevant financial relationships.
Robinderpal Sandhu indicated no relevant financial relationships.
Ezana Bekele indicated no relevant financial relationships.
Meghaben Kothari indicated no relevant financial relationships.
Ramnik K. Gill, MD, MS1, Robinderpal Sandhu, MD2, Ezana Bekele, MD1, Meghaben Kothari, MD1. P4017 - A Rare Case of Checkpoint Inhibitor Mediated Hepatitis in a Patient With Melanoma, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
