Tuesday Poster Session
Category: Colon
P3021 - Comparing Differential Dosing Regimens of Oral Sulfate Tablet Preparations: Impact on Fecal and Fluid Parameters
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
Has Audio
- MW
Matthew L. Walker, PhD
Braintree, A part of Sebela Pharmaceuticals
Braintree, MA
Presenting Author(s)
Matthew L. Walker, PhD1, Robert Bass, MD2
1Braintree, A part of Sebela Pharmaceuticals, Braintree, MA; 2ICON, San Antonio, TX
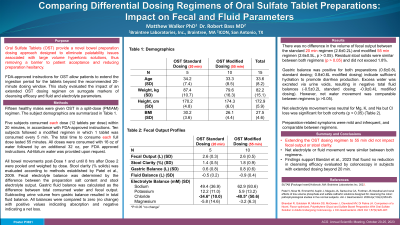
Introduction: Oral Sulfate Tablet (OST) bowel preparation eliminates palatability issues associated with large volume hypertonic solutions, thus removing a barrier to patient acceptance. FDA-approved instructions for OST allow patients to extend the ingestion period for the tablets beyond the recommended 20 minute dosing window. This study evaluated the impact of an extended OST dosing regimen on fecal, electrolyte and fluid parameters.
Methods: 15 healthy males (33.6±8.2 yoa) were given OST in a split-dose (PM/AM) regimen. 5 subjects consumed all tablets within 20 min, in accordance with FDA-approved instructions. 10 subjects followed a modified regimen (1 tablet every 5 min, 55 min total). Doses were consumed with 16 oz of water followed by an additional 32 oz of water. Ad-libitum water was provided upon request.
Bowel movements post-Dose 1 and until 8 hrs after Dose 2 were pooled and weighed by dose. Stool clarity (% solids) was evaluated according to methods established by Patel et al., 2010. Fecal electrolyte balance was determined by the difference between the preparation salt content and stool electrolyte output. Gastric fluid balance was calculated as the difference between total consumed water and fecal output. Subtracting urine volume from gastric balance resulted in total fluid balance. All balances were compared to zero (no change) with positive values indicating absorption and negative indicating a net loss.
Results: There was no difference in fecal output between the standard (2.6±0.2L) and modified regimens (2.6±0.5L, p >0.05). Residual stool solids were low (<1.8%) for both regimens (p >0.05).
Net fluid movement was comparable between regimens (p >0.05). The positive gastric balances (0.6±0.8L, standard dosing; 0.8±0.6L modified dosing) indicate sufficient hydration to promote diarrhea production. Excess water was excreted via urine voids, resulting in negative total fluid balances (-0.5±0.2L standard dosing; -0.9±0.4L modified dosing). Net electrolyte movement was neutral for Mg, K, and Na but Cl loss was significant for both regimens.
Preparation-related symptoms were mild and infrequent, and comparable between regimens.
Discussion: Modifying the dosing regimen of OST (1 tablet every 5 mins) does not impact fecal output or clarity. Additionally, there is no physiological difference in net electrolyte or fluid movement. These findings suggest that a modified regimen is expected to have similar cleansing efficacy to the standard dosing format.
Disclosures:
Matthew L. Walker, PhD1, Robert Bass, MD2. P3021 - Comparing Differential Dosing Regimens of Oral Sulfate Tablet Preparations: Impact on Fecal and Fluid Parameters, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Braintree, A part of Sebela Pharmaceuticals, Braintree, MA; 2ICON, San Antonio, TX
Introduction: Oral Sulfate Tablet (OST) bowel preparation eliminates palatability issues associated with large volume hypertonic solutions, thus removing a barrier to patient acceptance. FDA-approved instructions for OST allow patients to extend the ingestion period for the tablets beyond the recommended 20 minute dosing window. This study evaluated the impact of an extended OST dosing regimen on fecal, electrolyte and fluid parameters.
Methods: 15 healthy males (33.6±8.2 yoa) were given OST in a split-dose (PM/AM) regimen. 5 subjects consumed all tablets within 20 min, in accordance with FDA-approved instructions. 10 subjects followed a modified regimen (1 tablet every 5 min, 55 min total). Doses were consumed with 16 oz of water followed by an additional 32 oz of water. Ad-libitum water was provided upon request.
Bowel movements post-Dose 1 and until 8 hrs after Dose 2 were pooled and weighed by dose. Stool clarity (% solids) was evaluated according to methods established by Patel et al., 2010. Fecal electrolyte balance was determined by the difference between the preparation salt content and stool electrolyte output. Gastric fluid balance was calculated as the difference between total consumed water and fecal output. Subtracting urine volume from gastric balance resulted in total fluid balance. All balances were compared to zero (no change) with positive values indicating absorption and negative indicating a net loss.
Results: There was no difference in fecal output between the standard (2.6±0.2L) and modified regimens (2.6±0.5L, p >0.05). Residual stool solids were low (<1.8%) for both regimens (p >0.05).
Net fluid movement was comparable between regimens (p >0.05). The positive gastric balances (0.6±0.8L, standard dosing; 0.8±0.6L modified dosing) indicate sufficient hydration to promote diarrhea production. Excess water was excreted via urine voids, resulting in negative total fluid balances (-0.5±0.2L standard dosing; -0.9±0.4L modified dosing). Net electrolyte movement was neutral for Mg, K, and Na but Cl loss was significant for both regimens.
Preparation-related symptoms were mild and infrequent, and comparable between regimens.
Discussion: Modifying the dosing regimen of OST (1 tablet every 5 mins) does not impact fecal output or clarity. Additionally, there is no physiological difference in net electrolyte or fluid movement. These findings suggest that a modified regimen is expected to have similar cleansing efficacy to the standard dosing format.
Disclosures:
Matthew Walker: Braintree, A Part of Sebela – Employee.
Robert Bass: .
Matthew L. Walker, PhD1, Robert Bass, MD2. P3021 - Comparing Differential Dosing Regimens of Oral Sulfate Tablet Preparations: Impact on Fecal and Fluid Parameters, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
