Tuesday Poster Session
Category: Colon
P3027 - Implementation of Blood-Based Colorectal Cancer Screening: Real-World Clinical Experience
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
- VR
Victoria Raymond, MS
Guardant Health
Palo Alto, California
Presenting Author(s)
Victoria Raymond, MS1, Gail Foster, RN, BSN, MHS, MBA2, Yanyan Hong, PhD3, Theresa Hoang, PharmD3, Jiayue Liu, PhD4, Jordan Burke, PhD3, Sven Duenwald, 1, Darya Chudova, PhD1, Martina Lefterova, MD, PhD3, AmirAli Talasaz, PhD1
1Guardant Health, Palo Alto, CA; 2Guardant Health, Tyler, TX; 3Guardant Health, Redwood City, CA; 4Guardant Health, Seattle, WA
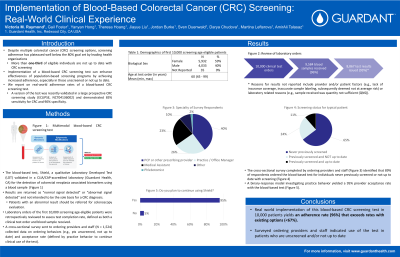
Introduction: Despite multiple colorectal cancer (CRC) screening options, screening adherence has plateaued well below the 80% goal set by leading health organizations, with more than one-third of eligible individuals not up to date. Implementation of a blood-based CRC screening test can enhance effectiveness of population-based screening programs by achieving increased adherence, especially in those unscreened or not up to date. We report on real-world adherence rates of a blood-based CRC screening test. A version of the test was recently validated in a large prospective CRC screening study (ECLIPSE, NCT04136002) and demonstrated 83% sensitivity and 90% specificity for CRC.
Methods: The blood-based test (Shield) is a qualitative Laboratory Developed Test (LDT) validated in a CLIA/CAP-accredited laboratory (Guardant Health, CA) for CRC screening in average risk, guideline recommended individuals, age 45+. Results are returned as “normal signal detected” or “abnormal signal detected” and not intended to be the sole basis for a CRC diagnosis. Patients (pts) with an abnormal result should be referred for colonoscopy evaluation. Laboratory orders of the first 10,000 screening age-eligible pts were retrospectively reviewed. Test completion is defined as both clinical test order and blood sample received. A cross-sectional survey sent to ordering providers and staff (N=1,524) collected data on ordering behaviors (e.g., pts unscreened, not up to date) and acceptance rate (defined by practice behavior to continue clinical use of the test).
Results: 9,584/10,000 pts completed the blood-based test, yielding a 96% adherence. 59% were female. Median age: 60 years (range: 45-99). 93% (N = 8,867) of tests were issued results. Reasons for results not reported include provider/pt factors (e.g., lack of insurance coverage, inaccurate sample labeling, subsequently deemed not at average risk) or laboratory related reasons (e.g., sample received was quantity not sufficient (QNS)). The cross-sectional survey completed by ordering providers and staff identified that 89% of respondents ordered the blood-based test for individuals never previously screened (65%) or not up to date with screening (24%). A binary-response model investigating practice behavior yielded a 95% provider acceptance rate with the blood-based test.
Discussion: Implementation of this blood-based CRC screening test in 10,000 pts yields an adherence rate (96%) that exceeds rates with existing options (< 67%).
Disclosures:
Victoria Raymond, MS1, Gail Foster, RN, BSN, MHS, MBA2, Yanyan Hong, PhD3, Theresa Hoang, PharmD3, Jiayue Liu, PhD4, Jordan Burke, PhD3, Sven Duenwald, 1, Darya Chudova, PhD1, Martina Lefterova, MD, PhD3, AmirAli Talasaz, PhD1. P3027 - Implementation of Blood-Based Colorectal Cancer Screening: Real-World Clinical Experience, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Guardant Health, Palo Alto, CA; 2Guardant Health, Tyler, TX; 3Guardant Health, Redwood City, CA; 4Guardant Health, Seattle, WA
Introduction: Despite multiple colorectal cancer (CRC) screening options, screening adherence has plateaued well below the 80% goal set by leading health organizations, with more than one-third of eligible individuals not up to date. Implementation of a blood-based CRC screening test can enhance effectiveness of population-based screening programs by achieving increased adherence, especially in those unscreened or not up to date. We report on real-world adherence rates of a blood-based CRC screening test. A version of the test was recently validated in a large prospective CRC screening study (ECLIPSE, NCT04136002) and demonstrated 83% sensitivity and 90% specificity for CRC.
Methods: The blood-based test (Shield) is a qualitative Laboratory Developed Test (LDT) validated in a CLIA/CAP-accredited laboratory (Guardant Health, CA) for CRC screening in average risk, guideline recommended individuals, age 45+. Results are returned as “normal signal detected” or “abnormal signal detected” and not intended to be the sole basis for a CRC diagnosis. Patients (pts) with an abnormal result should be referred for colonoscopy evaluation. Laboratory orders of the first 10,000 screening age-eligible pts were retrospectively reviewed. Test completion is defined as both clinical test order and blood sample received. A cross-sectional survey sent to ordering providers and staff (N=1,524) collected data on ordering behaviors (e.g., pts unscreened, not up to date) and acceptance rate (defined by practice behavior to continue clinical use of the test).
Results: 9,584/10,000 pts completed the blood-based test, yielding a 96% adherence. 59% were female. Median age: 60 years (range: 45-99). 93% (N = 8,867) of tests were issued results. Reasons for results not reported include provider/pt factors (e.g., lack of insurance coverage, inaccurate sample labeling, subsequently deemed not at average risk) or laboratory related reasons (e.g., sample received was quantity not sufficient (QNS)). The cross-sectional survey completed by ordering providers and staff identified that 89% of respondents ordered the blood-based test for individuals never previously screened (65%) or not up to date with screening (24%). A binary-response model investigating practice behavior yielded a 95% provider acceptance rate with the blood-based test.
Discussion: Implementation of this blood-based CRC screening test in 10,000 pts yields an adherence rate (96%) that exceeds rates with existing options (< 67%).
Disclosures:
Victoria Raymond: Guardant Health – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Gail Foster indicated no relevant financial relationships.
Yanyan Hong indicated no relevant financial relationships.
Theresa Hoang: Guardant Health – Employee, Stock-publicly held company(excluding mutual/index funds).
Jiayue Liu: Guardant – Employee.
Jordan Burke: Guardant Health – Employee, Stock-publicly held company(excluding mutual/index funds).
Sven Duenwald: Guardant Health – Employee.
Darya Chudova: Guardant Health – Employee, Intellectual Property/Patents, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Martina Lefterova: Guardant Health – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
AmirAli Talasaz: Guardant Health – Employee, Stock Options.
Victoria Raymond, MS1, Gail Foster, RN, BSN, MHS, MBA2, Yanyan Hong, PhD3, Theresa Hoang, PharmD3, Jiayue Liu, PhD4, Jordan Burke, PhD3, Sven Duenwald, 1, Darya Chudova, PhD1, Martina Lefterova, MD, PhD3, AmirAli Talasaz, PhD1. P3027 - Implementation of Blood-Based Colorectal Cancer Screening: Real-World Clinical Experience, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
