Tuesday Poster Session
Category: Colon
P3132 - Belatecept-Induced Colitis in a Patient with Graft Rejection
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
Has Audio

Apoorva Doshi
Seth Gordhandas Sundardas Medical College and KEM Hospital
Bharuch, Gujarat, India
Presenting Author(s)
Apoorva Doshi, 1, Lasha Gogokhia, MD2, Nicole Mendelson, MD3, David Wan, BS, MD4
1Seth Gordhandas Sundardas Medical College and KEM Hospital, Mumbai, Maharashtra, India; 2New York-Presbyterian Hospital/Weill Cornell Medical Center, New York, NY; 3Weill Cornell Medicine/New York Presbyterian, New York, NY; 4Weill Cornell Medicine, New York, NY
Introduction: Belatecept is a cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) Ig fusion protein indicated for kidney transplant rejection prophylaxis and has the potential to cause immune dysregulation. Here we report a case of Belatecept-induced colitis in a patient with chronic kidney graft rejection.
Case Description/Methods: 33-year-old female with previously diagnosed ulcerative colitis (UC) and chronic kidney graft rejection presented with abdominal pain, bloody diarrhea and fever. She was diagnosed with UC 4 years ago and was started on budesonide. Her previous colonoscopy, 8 months ago, showed Mayo grade 1 proctosigmoiditis. She was on prednisone and Belatecept due to renal transplant but developed chronic graft rejection requiring dialysis. On presentation, patient was febrile, otherwise her vitals were stable. Physical examination revealed mild left lower quadrant tenderness and bright red blood per rectum. Labs were notable for Hb 9.1 g/dL, WBC 10,000/dL, creatinine 7 mg/dL, and elevated CRP/ESR. CT Abdomen revealed colonic wall thickening and fat stranding (Image 1.A). Infectious workup was negative. Flexible sigmoidoscopy revealed Mayo grade 1 proctosigmoiditis (Image 1.B). Histology (Image 1.C-D) revealed chronic active colitis, crypt dropout and apoptotic epithelial cells indicating a medication induced injury rather than a UC flare. Belatecept was suspected to be the culprit and was discontinued. After a partial response to IV methylprednisolone 60 mg, a higher dose resulted in clinical resolution. She was put on a steroid taper and discharged.
Discussion: Here we report a case of Belatecept-induced colitis in a patient with ESRD after renal transplant. Belatecept is a recombinant CTLA-4 Ig fusion protein that prevents CD28 T-cell co-stimulation by binding to CD80/CD86 on antigen presenting cells. By selectively modulating T-cell activation it could cause immune dysregulation and colitis. Its role in colitis is further strengthened by the clinical improvement with high dose steroids and drug discontinuation. So far four case reports have been published implicating CTLA-4 Ig agents in developing colitis, with varying presentations. Similarities to our case included a considerable time gap between starting the drug and development of colitis, and a previous diagnosis of UC or Crohn's disease. These cases lend evidence for CTLA-4 Ig agents causing immune dysregulation resulting in colitis. Clinicians should consider this potential adverse effect in patients on CTLA-4 Ig agents.

Disclosures:
Apoorva Doshi, 1, Lasha Gogokhia, MD2, Nicole Mendelson, MD3, David Wan, BS, MD4. P3132 - Belatecept-Induced Colitis in a Patient with Graft Rejection, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Seth Gordhandas Sundardas Medical College and KEM Hospital, Mumbai, Maharashtra, India; 2New York-Presbyterian Hospital/Weill Cornell Medical Center, New York, NY; 3Weill Cornell Medicine/New York Presbyterian, New York, NY; 4Weill Cornell Medicine, New York, NY
Introduction: Belatecept is a cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) Ig fusion protein indicated for kidney transplant rejection prophylaxis and has the potential to cause immune dysregulation. Here we report a case of Belatecept-induced colitis in a patient with chronic kidney graft rejection.
Case Description/Methods: 33-year-old female with previously diagnosed ulcerative colitis (UC) and chronic kidney graft rejection presented with abdominal pain, bloody diarrhea and fever. She was diagnosed with UC 4 years ago and was started on budesonide. Her previous colonoscopy, 8 months ago, showed Mayo grade 1 proctosigmoiditis. She was on prednisone and Belatecept due to renal transplant but developed chronic graft rejection requiring dialysis. On presentation, patient was febrile, otherwise her vitals were stable. Physical examination revealed mild left lower quadrant tenderness and bright red blood per rectum. Labs were notable for Hb 9.1 g/dL, WBC 10,000/dL, creatinine 7 mg/dL, and elevated CRP/ESR. CT Abdomen revealed colonic wall thickening and fat stranding (Image 1.A). Infectious workup was negative. Flexible sigmoidoscopy revealed Mayo grade 1 proctosigmoiditis (Image 1.B). Histology (Image 1.C-D) revealed chronic active colitis, crypt dropout and apoptotic epithelial cells indicating a medication induced injury rather than a UC flare. Belatecept was suspected to be the culprit and was discontinued. After a partial response to IV methylprednisolone 60 mg, a higher dose resulted in clinical resolution. She was put on a steroid taper and discharged.
Discussion: Here we report a case of Belatecept-induced colitis in a patient with ESRD after renal transplant. Belatecept is a recombinant CTLA-4 Ig fusion protein that prevents CD28 T-cell co-stimulation by binding to CD80/CD86 on antigen presenting cells. By selectively modulating T-cell activation it could cause immune dysregulation and colitis. Its role in colitis is further strengthened by the clinical improvement with high dose steroids and drug discontinuation. So far four case reports have been published implicating CTLA-4 Ig agents in developing colitis, with varying presentations. Similarities to our case included a considerable time gap between starting the drug and development of colitis, and a previous diagnosis of UC or Crohn's disease. These cases lend evidence for CTLA-4 Ig agents causing immune dysregulation resulting in colitis. Clinicians should consider this potential adverse effect in patients on CTLA-4 Ig agents.

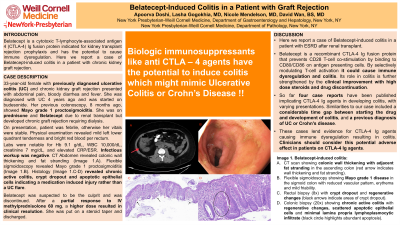
Figure: Image 1. Belatecept-induced colitis:
A. CT scan showing colonic wall thickening with adjacent fat stranding in the ascending colon (red arrow indicates wall thickening and fat stranding);
B. Flexible sigmoidoscopy showing Mayo grade 1 disease in the sigmoid colon with reduced vascular pattern, erythema and mild friability;
C. Rectal biopsy (8x) with crypt dropout and regenerative changes (black arrows indicate areas of crypt dropout);
D.Colonic biopsy (20x) showing chronic active colitis with regenerative changes, scattered apoptotic epithelial cells and minimal lamina propria lymphoplasmocytic infiltrate (black circle highlights abundant apoptosis).
A. CT scan showing colonic wall thickening with adjacent fat stranding in the ascending colon (red arrow indicates wall thickening and fat stranding);
B. Flexible sigmoidoscopy showing Mayo grade 1 disease in the sigmoid colon with reduced vascular pattern, erythema and mild friability;
C. Rectal biopsy (8x) with crypt dropout and regenerative changes (black arrows indicate areas of crypt dropout);
D.Colonic biopsy (20x) showing chronic active colitis with regenerative changes, scattered apoptotic epithelial cells and minimal lamina propria lymphoplasmocytic infiltrate (black circle highlights abundant apoptosis).
Disclosures:
Apoorva Doshi indicated no relevant financial relationships.
Lasha Gogokhia indicated no relevant financial relationships.
Nicole Mendelson indicated no relevant financial relationships.
David Wan indicated no relevant financial relationships.
Apoorva Doshi, 1, Lasha Gogokhia, MD2, Nicole Mendelson, MD3, David Wan, BS, MD4. P3132 - Belatecept-Induced Colitis in a Patient with Graft Rejection, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.

