Tuesday Poster Session
Category: Colorectal Cancer Prevention
P3194 - Cost-Effectiveness of Mt-sDNA vs Fecal Immunochemical Test for Colorectal Cancer Screening in a Medicare Population
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
Has Audio

Derek W. Ebner, MD
Mayo Clinic
Rochester, Minnesota
Presenting Author(s)
Award: Presidential Poster Award
Derek W.. Ebner, MD1, John Kisiel, MD1, Vahab Vahdat, PhD2, Lesley-Ann Miller-Wilson, PhD2, Nancy El Hoyek, PharmD2, Paul J.. Limburg, MD2
1Mayo Clinic, Rochester, MN; 2Exact Sciences, Madison, WI
Introduction: Multi-target stool DNA (mt-sDNA) test has a statistically higher sensitivity for colorectal cancer (CRC) detection than fecal immunotherapy test (FIT), particularly in detection of precancerous lesions. Both tests are recommended by US CRC screening guidelines. FIT became widely adopted in light of improved sensitivity over guaiac fecal occult testing and has a low test cost. However, low adherence to annual FIT screening in the US reduces projected health outcome impacts. Unlike mt-sDNA, FIT does not include patient programs to improve adherence; the costs for these programs can vary. To evaluate the integration of adherence programs with FIT, this analysis estimated cost-effectiveness of mt-sDNA vs FIT at varying FIT test and FIT program costs for a Medicare population.
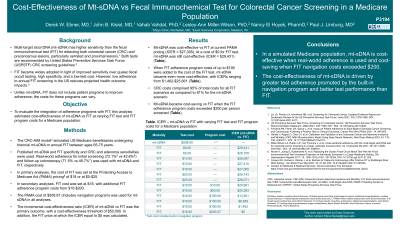
Methods: The CRC-AIM model simulated US Medicare beneficiaries undergoing triennial mt-sDNA or annual FIT between ages 65-75 years. Published mt-sDNA and FIT specificity and CRC and adenoma sensitivities were used. Real-world adherence for initial screening (72.1% vs 42.6%) and follow-up colonoscopy (71.5% vs 46.7%) was used with mt-sDNA and FIT, respectively. In primary analyses, the cost of FIT was set at the Protecting Access to Medicare Act (PAMA) pricing of $18 or at $0-$25. In secondary analyses, FIT cost was set at $18, with additional FIT adherence program costs from $10-$200. The PAMA cost of $508 (includes navigation program) was used for mt-sDNA in all analyses. The incremental cost-effectiveness ratio (ICER) of mt-sDNA vs FIT was the primary outcome, with a cost-effectiveness threshold of $50,000.
Results: Mt-sDNA was cost-effective vs FIT at current PAMA pricing (ICER = $27,005). At a cost of $0 for FIT test, mt-sDNA was still cost-effective (ICER = $29,431). When FIT adherence program costs of up to $190 were added to the cost of the FIT test, mt-sDNA became even more cost-effective, with ICERs ranging from $1,462-$25,661. Mt-sDNA became cost-saving vs FIT when the FIT adherence program costs exceeded $200 per person screened.
Discussion: While previous analysis showed that mt-sDNA is not cost-effective vs FIT at unrealistic perfect adherence, in a simulated Medicare population, mt-sDNA is cost-effective when real-world adherence is used and cost-saving when navigation cost is added to FIT programs. The cost-effectiveness of mt-sDNA is driven by greater test adherence promoted by the built-in navigation program and better test performance than FIT.
Disclosures:
Derek W.. Ebner, MD1, John Kisiel, MD1, Vahab Vahdat, PhD2, Lesley-Ann Miller-Wilson, PhD2, Nancy El Hoyek, PharmD2, Paul J.. Limburg, MD2. P3194 - Cost-Effectiveness of Mt-sDNA vs Fecal Immunochemical Test for Colorectal Cancer Screening in a Medicare Population, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
Derek W.. Ebner, MD1, John Kisiel, MD1, Vahab Vahdat, PhD2, Lesley-Ann Miller-Wilson, PhD2, Nancy El Hoyek, PharmD2, Paul J.. Limburg, MD2
1Mayo Clinic, Rochester, MN; 2Exact Sciences, Madison, WI
Introduction: Multi-target stool DNA (mt-sDNA) test has a statistically higher sensitivity for colorectal cancer (CRC) detection than fecal immunotherapy test (FIT), particularly in detection of precancerous lesions. Both tests are recommended by US CRC screening guidelines. FIT became widely adopted in light of improved sensitivity over guaiac fecal occult testing and has a low test cost. However, low adherence to annual FIT screening in the US reduces projected health outcome impacts. Unlike mt-sDNA, FIT does not include patient programs to improve adherence; the costs for these programs can vary. To evaluate the integration of adherence programs with FIT, this analysis estimated cost-effectiveness of mt-sDNA vs FIT at varying FIT test and FIT program costs for a Medicare population.
Methods: The CRC-AIM model simulated US Medicare beneficiaries undergoing triennial mt-sDNA or annual FIT between ages 65-75 years. Published mt-sDNA and FIT specificity and CRC and adenoma sensitivities were used. Real-world adherence for initial screening (72.1% vs 42.6%) and follow-up colonoscopy (71.5% vs 46.7%) was used with mt-sDNA and FIT, respectively. In primary analyses, the cost of FIT was set at the Protecting Access to Medicare Act (PAMA) pricing of $18 or at $0-$25. In secondary analyses, FIT cost was set at $18, with additional FIT adherence program costs from $10-$200. The PAMA cost of $508 (includes navigation program) was used for mt-sDNA in all analyses. The incremental cost-effectiveness ratio (ICER) of mt-sDNA vs FIT was the primary outcome, with a cost-effectiveness threshold of $50,000.
Results: Mt-sDNA was cost-effective vs FIT at current PAMA pricing (ICER = $27,005). At a cost of $0 for FIT test, mt-sDNA was still cost-effective (ICER = $29,431). When FIT adherence program costs of up to $190 were added to the cost of the FIT test, mt-sDNA became even more cost-effective, with ICERs ranging from $1,462-$25,661. Mt-sDNA became cost-saving vs FIT when the FIT adherence program costs exceeded $200 per person screened.
Discussion: While previous analysis showed that mt-sDNA is not cost-effective vs FIT at unrealistic perfect adherence, in a simulated Medicare population, mt-sDNA is cost-effective when real-world adherence is used and cost-saving when navigation cost is added to FIT programs. The cost-effectiveness of mt-sDNA is driven by greater test adherence promoted by the built-in navigation program and better test performance than FIT.
Disclosures:
Derek Ebner: Exact Sciences – Consultant.
John Kisiel: Exact Sciences – Consultant, Grant/Research Support, Intellectual Property/Patents, Royalties.
Vahab Vahdat: Exact Sciences – Employee, Stock-publicly held company(excluding mutual/index funds).
Lesley-Ann Miller-Wilson: Exact Sciences – Employee, Stock-publicly held company(excluding mutual/index funds).
Nancy El Hoyek: Exact Sciences – Employee, Stock-publicly held company(excluding mutual/index funds).
Paul Limburg: Exact Sciences – Consultant, Employee, Grant/Research Support, Intellectual Property/Patents, Stock-publicly held company(excluding mutual/index funds). Ironwood Pharmaceuticals – Grant/Research Support. Thorne Research – Grant/Research Support.
Derek W.. Ebner, MD1, John Kisiel, MD1, Vahab Vahdat, PhD2, Lesley-Ann Miller-Wilson, PhD2, Nancy El Hoyek, PharmD2, Paul J.. Limburg, MD2. P3194 - Cost-Effectiveness of Mt-sDNA vs Fecal Immunochemical Test for Colorectal Cancer Screening in a Medicare Population, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.


