Tuesday Poster Session
Category: Colorectal Cancer Prevention
P3203 - Molecular Tumor Testing on Colorectal Adenocarcinoma Specimens in a Large Community-Based Healthcare System
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
Has Audio

David H. Kruchko, DO
Advocate Aurora Health
Chicago, IL
Presenting Author(s)
David H. Kruchko, DO1, Sareena Ali, DO2, Madeline Sesselmann, DO1, Mahbubul Hasan, MS1, Imad Almanaseer, MD1, Eli D. Ehrenpreis, MD3
1Advocate Aurora Health, Chicago, IL; 2Advocate Lutheran General Hospital, Park Ridge, IL; 3Advocate Lutheran General Hospital, Skokie, IL
Introduction: In 2015, the National Comprehensive Cancer Network (NCCN) published a guideline recommending universal molecular tumor testing with immunohistochemical staining (IHC) for microsatellite instability (MSI) on all colorectal cancer (CRC) specimens to identify patients at high risk for inherited cancer syndromes. Data from university hospitals demonstrates a high uptake of molecular tumor testing reported to be 93%. Our study aims to determine the frequency of IHC testing in a large community-based health system and to determine factors associated with ordering of IHC testing.
Methods: The pathology database system (CoPath) for patients in the Advocate Aurora Health (AAH) for CRC specimens was evaluated for 01/01/2016 to 12/31/2020. Data collected for each pathology specimen included the ordering pathologist, location of CRC (i.e. ascending colon), patient medical record number (MRN), and whether IHC testing was performed. Data were tabulated manually into a secure database. MRNs were used to eliminate duplicate specimens. Names of pathologists were changed to unique numbers. Pathologist practice location (within the AAH system) was determined. Descriptive statistics were presented as means and standard deviations for continuous data and as counts and percentages for dichotomous and categorical data. Statistical significance of categorical variables was tested by Pearson chi-square or Fisher's exact test for logistic regression categorical variables.
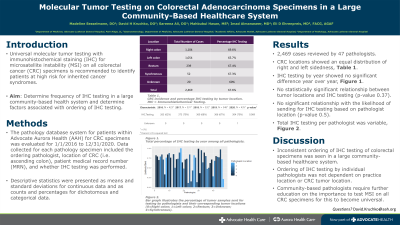
Results: A total of 2,469 cases of CRC reviewed by 47 pathologists were identified within AAH system for the selected five-year period. CRC locations showed an equal distribution of right and left sidedness, results are shown in Table 1. IHC testing by year showed no significant difference year over year, results can be found in Figure 1. There was no statistical significance relationship between tumor locations and IHC testing (p-value 0.37), and likelihood of sending for IHC testing based on pathologist location (p-value 0.5) in Figure 2 with total IHC testing per pathologist found in Figure 3.
Discussion: Inconsistent ordering of IHC testing of colorectal specimens was seen in a large community-based healthcare system. Ordering of IHC testing by individual pathologists was not dependent on practice location or CRC tumor location. Education on the importance of universal IHC testing for CRC specimens during this time period is suggested.

Disclosures:
David H. Kruchko, DO1, Sareena Ali, DO2, Madeline Sesselmann, DO1, Mahbubul Hasan, MS1, Imad Almanaseer, MD1, Eli D. Ehrenpreis, MD3. P3203 - Molecular Tumor Testing on Colorectal Adenocarcinoma Specimens in a Large Community-Based Healthcare System, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Advocate Aurora Health, Chicago, IL; 2Advocate Lutheran General Hospital, Park Ridge, IL; 3Advocate Lutheran General Hospital, Skokie, IL
Introduction: In 2015, the National Comprehensive Cancer Network (NCCN) published a guideline recommending universal molecular tumor testing with immunohistochemical staining (IHC) for microsatellite instability (MSI) on all colorectal cancer (CRC) specimens to identify patients at high risk for inherited cancer syndromes. Data from university hospitals demonstrates a high uptake of molecular tumor testing reported to be 93%. Our study aims to determine the frequency of IHC testing in a large community-based health system and to determine factors associated with ordering of IHC testing.
Methods: The pathology database system (CoPath) for patients in the Advocate Aurora Health (AAH) for CRC specimens was evaluated for 01/01/2016 to 12/31/2020. Data collected for each pathology specimen included the ordering pathologist, location of CRC (i.e. ascending colon), patient medical record number (MRN), and whether IHC testing was performed. Data were tabulated manually into a secure database. MRNs were used to eliminate duplicate specimens. Names of pathologists were changed to unique numbers. Pathologist practice location (within the AAH system) was determined. Descriptive statistics were presented as means and standard deviations for continuous data and as counts and percentages for dichotomous and categorical data. Statistical significance of categorical variables was tested by Pearson chi-square or Fisher's exact test for logistic regression categorical variables.
Results: A total of 2,469 cases of CRC reviewed by 47 pathologists were identified within AAH system for the selected five-year period. CRC locations showed an equal distribution of right and left sidedness, results are shown in Table 1. IHC testing by year showed no significant difference year over year, results can be found in Figure 1. There was no statistical significance relationship between tumor locations and IHC testing (p-value 0.37), and likelihood of sending for IHC testing based on pathologist location (p-value 0.5) in Figure 2 with total IHC testing per pathologist found in Figure 3.
Discussion: Inconsistent ordering of IHC testing of colorectal specimens was seen in a large community-based healthcare system. Ordering of IHC testing by individual pathologists was not dependent on practice location or CRC tumor location. Education on the importance of universal IHC testing for CRC specimens during this time period is suggested.

Figure: Figure 1. Total percentage of IHC testing by year.
Figure 2. Mean likelihood of sending for IHC testing based on pathologist location.
Figure 3. Bar graph of all pathologist’s percentage sent with cohort color sorting.
Figure 2. Mean likelihood of sending for IHC testing based on pathologist location.
Figure 3. Bar graph of all pathologist’s percentage sent with cohort color sorting.
Right colon | 1,104 |
Left colon | 1,054 |
Rectum | 239 |
Synchronous | 52 |
Unknown | 20 |
Total | 2,469 |
Disclosures:
David Kruchko indicated no relevant financial relationships.
Sareena Ali indicated no relevant financial relationships.
Madeline Sesselmann indicated no relevant financial relationships.
Mahbubul Hasan indicated no relevant financial relationships.
Imad Almanaseer indicated no relevant financial relationships.
Eli Ehrenpreis: E2Bio Life Sciences – Intellectual Property/Patents, Owner/Ownership Interest, Stock-privately held company. G.I. Pharmaceuticals, Inc. – Intellectual Property/Patents, Owner/Ownership Interest. Level Ex – Consultant.
David H. Kruchko, DO1, Sareena Ali, DO2, Madeline Sesselmann, DO1, Mahbubul Hasan, MS1, Imad Almanaseer, MD1, Eli D. Ehrenpreis, MD3. P3203 - Molecular Tumor Testing on Colorectal Adenocarcinoma Specimens in a Large Community-Based Healthcare System, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.

