Tuesday Poster Session
Category: Functional Bowel Disease
P3374 - The Efficacy of Treatments for Bile Acid Diarrhea: A Systematic Review and Meta-Analysis
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
Has Audio
- SD
Saam Dilmaghani, MD, MPH
Mayo Clinic
Rochester, MN
Presenting Author(s)
Award: Presidential Poster Award
Saam Dilmaghani, MD, MPH, Joelle BouSaba, MD, Larry Prokop, MLS, M. Hassan Murad, MD, MPH, Michael Camilleri, MD, DSc
Mayo Clinic, Rochester, MN
Introduction: Irritable bowel syndrome (IBS) affects up to 15% of the population. Among pathophysiological mechanisms for diarrhea-predominant IBS (IBS-D), one-third are due to bile acid (BA) diarrhea (BAD). The mainstay of treatment is bile acid sequestrants (BAS); yet the efficacy of BA-targeted medications is limited to case series or small randomized-controlled trials (RCT).
Methods: We conducted a systematic review and meta-analysis (SRMA) of blinded RCTs comparing BA-targeted therapies to placebo using a comprehensive search in any language of databases (Ovid MEDLINE(R), Ovid EMBASE, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, and Scopus) from inception to 1/18/2023. Inclusion required objective diagnosis of BAD (based on serum or fecal biochemical parameters, or 75SeHCAT retention). Endpoints included changes to stool frequency, consistency, and quality of life (QOL). Two independent reviewers screened abstracts for full text review. Data from included studies were extracted using a pre-specified form.
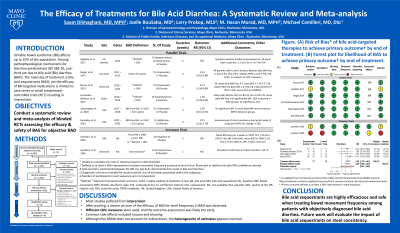
Results: Database search resulted in 1143 citations. 186 publications passed screening. Ultimately, 9 studies involving 232 patients were included in qualitative review and summarized in the Table. Seven were parallel arm and 2 crossover trials. All trials were from the USA and Europe. BA therapies evaluated were cholestyramine, colesevelam, tropifexor, and aldafermin. Most studies used 75SeHCAT < 10% for the diagnosis of BAD and assessed outcomes at 1-4 weeks. Study participants were 40-60 years old, 40-80% female, and BMI 25-35 kg/m2. The most consistently reported outcome across trials was a 30% improvement in stool frequency (“primary outcome”). The risk of bias for most studies was low. Meta-analysis focusing on BAS is summarized in the Figure. Meta-analysis of parallel design trials comparing a BAS to placebo demonstrated a clinically significant reduction in stool frequency with relative risk 2.80 (95% CI: 1.68-4.67), no heterogeneity (I2=0%) and with a moderate certainty of evidence. Of 5 studies that evaluated QOL, 3 found no difference, 1 improvement in both groups, and 1 greater improvement in the intervention arm.
Discussion: This comprehensive SRMA increases the precision of estimates derived from individual studies and quantifies the significant and rapid improvement in stool frequency with BA-targeted therapies in patients with an objective diagnosis of BAD.

Disclosures:
Saam Dilmaghani, MD, MPH, Joelle BouSaba, MD, Larry Prokop, MLS, M. Hassan Murad, MD, MPH, Michael Camilleri, MD, DSc. P3374 - The Efficacy of Treatments for Bile Acid Diarrhea: A Systematic Review and Meta-Analysis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
Saam Dilmaghani, MD, MPH, Joelle BouSaba, MD, Larry Prokop, MLS, M. Hassan Murad, MD, MPH, Michael Camilleri, MD, DSc
Mayo Clinic, Rochester, MN
Introduction: Irritable bowel syndrome (IBS) affects up to 15% of the population. Among pathophysiological mechanisms for diarrhea-predominant IBS (IBS-D), one-third are due to bile acid (BA) diarrhea (BAD). The mainstay of treatment is bile acid sequestrants (BAS); yet the efficacy of BA-targeted medications is limited to case series or small randomized-controlled trials (RCT).
Methods: We conducted a systematic review and meta-analysis (SRMA) of blinded RCTs comparing BA-targeted therapies to placebo using a comprehensive search in any language of databases (Ovid MEDLINE(R), Ovid EMBASE, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, and Scopus) from inception to 1/18/2023. Inclusion required objective diagnosis of BAD (based on serum or fecal biochemical parameters, or 75SeHCAT retention). Endpoints included changes to stool frequency, consistency, and quality of life (QOL). Two independent reviewers screened abstracts for full text review. Data from included studies were extracted using a pre-specified form.
Results: Database search resulted in 1143 citations. 186 publications passed screening. Ultimately, 9 studies involving 232 patients were included in qualitative review and summarized in the Table. Seven were parallel arm and 2 crossover trials. All trials were from the USA and Europe. BA therapies evaluated were cholestyramine, colesevelam, tropifexor, and aldafermin. Most studies used 75SeHCAT < 10% for the diagnosis of BAD and assessed outcomes at 1-4 weeks. Study participants were 40-60 years old, 40-80% female, and BMI 25-35 kg/m2. The most consistently reported outcome across trials was a 30% improvement in stool frequency (“primary outcome”). The risk of bias for most studies was low. Meta-analysis focusing on BAS is summarized in the Figure. Meta-analysis of parallel design trials comparing a BAS to placebo demonstrated a clinically significant reduction in stool frequency with relative risk 2.80 (95% CI: 1.68-4.67), no heterogeneity (I2=0%) and with a moderate certainty of evidence. Of 5 studies that evaluated QOL, 3 found no difference, 1 improvement in both groups, and 1 greater improvement in the intervention arm.
Discussion: This comprehensive SRMA increases the precision of estimates derived from individual studies and quantifies the significant and rapid improvement in stool frequency with BA-targeted therapies in patients with an objective diagnosis of BAD.

Figure: Figure. (A) Risk of bias* of bile acid-targeted therapies to achieve primary outcome† by end of treatment. (B) Forest plot for likelihood of BAS to achieve primary outcome† by end of treatment.
* As adapted from the Revised Cochrane Risk of Bias Tool for Randomized Trials (RoB2) found at https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials.
† Primary outcome defined as at least a 30% improvement in stool frequency
* As adapted from the Revised Cochrane Risk of Bias Tool for Randomized Trials (RoB2) found at https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials.
† Primary outcome defined as at least a 30% improvement in stool frequency
Disclosures:
Saam Dilmaghani indicated no relevant financial relationships.
Joelle BouSaba indicated no relevant financial relationships.
Larry Prokop indicated no relevant financial relationships.
M. Hassan Murad indicated no relevant financial relationships.
Michael Camilleri: NGM Biopharmaceuticals – Grant/Research Support.
Saam Dilmaghani, MD, MPH, Joelle BouSaba, MD, Larry Prokop, MLS, M. Hassan Murad, MD, MPH, Michael Camilleri, MD, DSc. P3374 - The Efficacy of Treatments for Bile Acid Diarrhea: A Systematic Review and Meta-Analysis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.

