Tuesday Poster Session
Category: Functional Bowel Disease
P3379 - Efficacy of Different Activation Modes of the Vibrating Capsule in Patients With Chronic Idiopathic Constipation
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
- WC
William D. Chey, MD, FACG
University of Michigan
Ann Arbor, Michigan
Presenting Author(s)
William D. Chey, MD, FACG1, Satish SC. Rao, MD, PhD2, Eamonn M. Quigley, MD, MACG3, Anthony Lembo, MD4, Bryan Curtin, MD, MHSc5, Christine L. Frissora, MD6, Darren M. Brenner, MD7
1University of Michigan, Ann Arbor, MI; 2Augusta University Medical Center, Augusta, GA; 3Houston Methodist Hospital, Houston, TX; 4Digestive Disease and Surgery Institute, Cleveland Clinic, Cleveland, OH; 5Mercy Medical Center, Baltimore, MD; 6Weill Cornell Medical College, New York, NY; 7Northwestern University Feinberg School of Medicine, Chicago, IL
Introduction: Current clinical practice guidelines for patients with Chronic Idiopathic Constipation (CIC) recommend empiric medical therapy in a tiered approach largely driven by cost and access rather than underlying pathophysiology. In a phase III study with the vibrating capsule (VC), 2 different activation modes vs placebo were evaluated until a planned interim analysis when the more effective of the 2 activation modes was identified and used to complete the trial. The aim of this post-hoc analysis was to compare the efficacy of Mode 1 vs Mode 2 vs Placebo.
Methods: We conducted a post-hoc analysis from the phase 3, multicenter, randomized, double-blind, placebo-controlled, 8-week trial of a VC (Vibrant ®, Yokneam, Israel) in patients with CIC, and compared the efficacy of Mode 1 (VC stimulation during second half of the day) vs Mode 2 (VC stimulation during first half of the day). Capsules were taken at 10pm. We evaluated the pre-programmed time of activation and its efficacy via responder rates of CSBMs (defined as % subjects with increases of at least 1 or at least 2 weekly CSBMs over baseline for 75% of treatment weeks). Mode 1 included 41 subjects and Mode 2 included 35 subjects, while the placebo arm included 40 subjects.
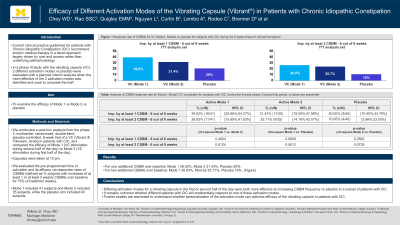
Results: Responders rates were as follows: For one additional CSBM over baseline: Mode 1 39.02%, Mode 2 31.43%, Placebo 20%. For two additional CSBMs over baseline: Mode 1 26.83%, Mode 2 25.71%, Placebo 10%. (Figure).
Discussion: Differing activation modes for a vibrating capsule in the first or second half of the day were both more effective at increasing CSBM frequency vs placebo in patients with CIC. It remains unknown whether different patients with CIC will preferentially respond to one of these activation modes. Further studies are warranted to understand whether personalization of the activation mode can optimize efficacy of the vibrating capsule in patients with CIC.

Disclosures:
William D. Chey, MD, FACG1, Satish SC. Rao, MD, PhD2, Eamonn M. Quigley, MD, MACG3, Anthony Lembo, MD4, Bryan Curtin, MD, MHSc5, Christine L. Frissora, MD6, Darren M. Brenner, MD7. P3379 - Efficacy of Different Activation Modes of the Vibrating Capsule in Patients With Chronic Idiopathic Constipation, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1University of Michigan, Ann Arbor, MI; 2Augusta University Medical Center, Augusta, GA; 3Houston Methodist Hospital, Houston, TX; 4Digestive Disease and Surgery Institute, Cleveland Clinic, Cleveland, OH; 5Mercy Medical Center, Baltimore, MD; 6Weill Cornell Medical College, New York, NY; 7Northwestern University Feinberg School of Medicine, Chicago, IL
Introduction: Current clinical practice guidelines for patients with Chronic Idiopathic Constipation (CIC) recommend empiric medical therapy in a tiered approach largely driven by cost and access rather than underlying pathophysiology. In a phase III study with the vibrating capsule (VC), 2 different activation modes vs placebo were evaluated until a planned interim analysis when the more effective of the 2 activation modes was identified and used to complete the trial. The aim of this post-hoc analysis was to compare the efficacy of Mode 1 vs Mode 2 vs Placebo.
Methods: We conducted a post-hoc analysis from the phase 3, multicenter, randomized, double-blind, placebo-controlled, 8-week trial of a VC (Vibrant ®, Yokneam, Israel) in patients with CIC, and compared the efficacy of Mode 1 (VC stimulation during second half of the day) vs Mode 2 (VC stimulation during first half of the day). Capsules were taken at 10pm. We evaluated the pre-programmed time of activation and its efficacy via responder rates of CSBMs (defined as % subjects with increases of at least 1 or at least 2 weekly CSBMs over baseline for 75% of treatment weeks). Mode 1 included 41 subjects and Mode 2 included 35 subjects, while the placebo arm included 40 subjects.
Results: Responders rates were as follows: For one additional CSBM over baseline: Mode 1 39.02%, Mode 2 31.43%, Placebo 20%. For two additional CSBMs over baseline: Mode 1 26.83%, Mode 2 25.71%, Placebo 10%. (Figure).
Discussion: Differing activation modes for a vibrating capsule in the first or second half of the day were both more effective at increasing CSBM frequency vs placebo in patients with CIC. It remains unknown whether different patients with CIC will preferentially respond to one of these activation modes. Further studies are warranted to understand whether personalization of the activation mode can optimize efficacy of the vibrating capsule in patients with CIC.

Figure: Response rate of CSBMs for VC Mode 1, Mode 2 vs placebo for subjects with CIC during the 8-week phase III clinical trial period.
Disclosures:
William Chey: Abbvie – Consultant. Alnylam – Consultant. Ardelyx – Consultant. Biomerica – Consultant. Commonwealth Diagnostics International – Grant/Research Support. Digital Manometry – Intellectual Property/Patents. Gemelli – Consultant. Ironwood – Consultant. Isothrive – Consultant, Stock Options. Kiwi BioScience – Stock Options. Modify Health – Stock Options. My Nutrition Health – Intellectual Property/Patents. Nestle – Consultant. QOL Medical – Consultant, Grant/Research Support. Rectal Expulsion Device – Intellectual Property/Patents. Redhill – Consultant. Salix/Valeant – Consultant, Grant/Research Support. Takeda – Consultant. Urovant Sciences – Consultant. Vibrant – Consultant.
Satish Rao indicated no relevant financial relationships.
Eamonn Quigley: 4D Pharma – Consultant, Grant/Research Support. Cindome – Grant/Research Support. Novozymes – Consultant. Salix – Consultant. Takeda – Grant/Research Support. Vibrant – Advisor or Review Panel Member, Grant/Research Support.
Anthony Lembo: AEON Biopharma Inc. – Consultant. Alkermes – Consultant. Allakos – Consultant. Allurion – Stock Options. Anji Pharmaceuticals – Consultant. Arena Pharmaceuticals – Consultant. BioAmerica – Consultant. Bristol Myers Squibb – Stock Options. Gemelli Biotech – Consultant. Ironwood Pharmaceuticals – Consultant. Johnson & Johnson – Stock Options. Maunea Kea – Consultant. Neurogastrx, Inc. – Consultant. OrphoMed, Inc. – Consultant. Pfizer – Consultant. QOL Medical – Consultant. Shire, a Takeda company – Consultant. Takeda Pharmaceuticals – Consultant. Vibrant Pharma, Inc. – Consultant.
Bryan Curtin: Vibrant – Advisor or Review Panel Member.
Christine Frissora: Ardelyx – Consultant. Mahana Therapeutics – Consultant. QOL Medical – Consultant. Vibrant – Consultant.
Darren Brenner: AbbVie Inc. – Advisor or Review Panel Member, Consultant, Speakers Bureau. Alnylam – Advisor or Review Panel Member, Consultant, Speakers Bureau. Ardelyx – Advisor or Review Panel Member, Consultant, Speakers Bureau. Bayer – Advisor or Review Panel Member, Consultant, Speakers Bureau. Entrinsic Bioscience – Advisor or Review Panel Member, Consultant, Speakers Bureau. Gemelli Biotech – Advisor or Review Panel Member, Consultant, Speakers Bureau. International Foundation for Gastrointestinal Disorders (IFFGD) – Advisory Committee/Board Member. Ironwood Pharmaceuticals, Inc. – Advisor or Review Panel Member, Consultant, Speakers Bureau. Mahana – Advisor or Review Panel Member, Consultant, Speakers Bureau. Owlstone – Advisor or Review Panel Member, Consultant, Speakers Bureau. Redhill – Advisor or Review Panel Member, Consultant, Speakers Bureau. Salix – Advisor or Review Panel Member, Consultant, Speakers Bureau. Takeda – Advisor or Review Panel Member, Consultant, Speakers Bureau. Vibrant – Advisor or Review Panel Member, Consultant, Speakers Bureau.
William D. Chey, MD, FACG1, Satish SC. Rao, MD, PhD2, Eamonn M. Quigley, MD, MACG3, Anthony Lembo, MD4, Bryan Curtin, MD, MHSc5, Christine L. Frissora, MD6, Darren M. Brenner, MD7. P3379 - Efficacy of Different Activation Modes of the Vibrating Capsule in Patients With Chronic Idiopathic Constipation, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
