Tuesday Poster Session
Category: GI Bleeding
P3456 - Hemospray vs PuraStat for the Management of Acute Gastrointestinal Bleeding & Prophylaxis of Post-Polypectomy Bleeding: A Large US Academic Cancer Center Experience
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
Has Audio

Iyad S. Al-Bustami, MD
University of Texas Health Science Center at Houston
Houston, TX
Presenting Author(s)
Iyad S.. Al-Bustami, MD1, Ahmad Nakshabandi, MD2, Antonio Pizuorno Machado, MD3, Daniel J. Low, MD4, Emmanuel Coronel, MD4, Phillip Ge, MD4
1University of Texas Health Science Center at Houston, Houston, TX; 2UT MD Anderson Cancer Center, Houston, TX; 3University of Texas Health Science Center, Houston, TX; 4University of Texas MD Anderson Cancer Center, Houston, TX
Introduction: Endoscopic management and prevention of gastrointestinal bleeding encompasses a wide array of tools and techniques, including the recent introduction of topical hemostatic agents. We aimed to evaluate and compare our experience with the TC-325 hemostatic powder (Hemospray, Cook Medical) and the recently approved self-assembling peptide hemostatic gel (PuraStat, 3D-Matrix).
Methods: We conducted a retrospective analysis of all endoscopic procedures performed from 2018-2023 at a tertiary cancer center in which a topical hemostatic agent (gel or powder) was used. Indications for hemostatic agent use (HA) were subgrouped into therapeutic intent (TI) for gastrointestinal bleeding, and prophylactic intent (PI) following endoscopic tissue resection including endoscopic mucosal resection and endoscopic submucosal dissection. Primary outcomes were early and delayed bleeding events (BE). Patient baseline characteristics were obtained including Charlson comorbidity index (CMI), anticoagulation at the time of bleeding, baseline hemoglobin level, hemostatic approaches (hemostatic powder/ gel, single/ dual therapy), and frequency of bleeding events. For the TI group, early BE interval was defined from completion of the procedure until 7 days post procedure, and delayed BE was defined from day 7 onwards. For the PI group, early BE was defined from completion of the procedure up to 3 days post procedure, and delayed BE was defined from day 3 onwards.
Results: We identified a total of 123 cases which included 67 cases in the TI group and 56 cases in the PI group. All-comers analysis was conducted to reach statistical power, although individual subgroup analysis was also conducted. The mean CMI of patients who developed delayed BE was higher compared to patients with no delayed BE (8.25± 2.05 vs 5.4± 3.2, p=0.006). Overall, use of hemostatic gel was associated with lower delayed BE compared to hemostatic powder (12.5% vs 87.5%, p=0.02). At two weeks following hemostatic agent use, a total of 17.2% patients encountered early or delayed BE, including 8.8% in the gel group vs 30.2% in the powder group (p=0.005). When compared to early BE, delayed BE required more frequent endoscopic re-interventions (87.5% vs 20%, p< 0.001).
Discussion: Regardless of indication, hemostatic gel was associated with lower bleeding rates compared to hemostatic powder, and delayed BE required more frequent endoscopic re-interventions. Ongoing prospective studies will seek to further validate these findings.

Disclosures:
Iyad S.. Al-Bustami, MD1, Ahmad Nakshabandi, MD2, Antonio Pizuorno Machado, MD3, Daniel J. Low, MD4, Emmanuel Coronel, MD4, Phillip Ge, MD4. P3456 - Hemospray vs PuraStat for the Management of Acute Gastrointestinal Bleeding & Prophylaxis of Post-Polypectomy Bleeding: A Large US Academic Cancer Center Experience, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1University of Texas Health Science Center at Houston, Houston, TX; 2UT MD Anderson Cancer Center, Houston, TX; 3University of Texas Health Science Center, Houston, TX; 4University of Texas MD Anderson Cancer Center, Houston, TX
Introduction: Endoscopic management and prevention of gastrointestinal bleeding encompasses a wide array of tools and techniques, including the recent introduction of topical hemostatic agents. We aimed to evaluate and compare our experience with the TC-325 hemostatic powder (Hemospray, Cook Medical) and the recently approved self-assembling peptide hemostatic gel (PuraStat, 3D-Matrix).
Methods: We conducted a retrospective analysis of all endoscopic procedures performed from 2018-2023 at a tertiary cancer center in which a topical hemostatic agent (gel or powder) was used. Indications for hemostatic agent use (HA) were subgrouped into therapeutic intent (TI) for gastrointestinal bleeding, and prophylactic intent (PI) following endoscopic tissue resection including endoscopic mucosal resection and endoscopic submucosal dissection. Primary outcomes were early and delayed bleeding events (BE). Patient baseline characteristics were obtained including Charlson comorbidity index (CMI), anticoagulation at the time of bleeding, baseline hemoglobin level, hemostatic approaches (hemostatic powder/ gel, single/ dual therapy), and frequency of bleeding events. For the TI group, early BE interval was defined from completion of the procedure until 7 days post procedure, and delayed BE was defined from day 7 onwards. For the PI group, early BE was defined from completion of the procedure up to 3 days post procedure, and delayed BE was defined from day 3 onwards.
Results: We identified a total of 123 cases which included 67 cases in the TI group and 56 cases in the PI group. All-comers analysis was conducted to reach statistical power, although individual subgroup analysis was also conducted. The mean CMI of patients who developed delayed BE was higher compared to patients with no delayed BE (8.25± 2.05 vs 5.4± 3.2, p=0.006). Overall, use of hemostatic gel was associated with lower delayed BE compared to hemostatic powder (12.5% vs 87.5%, p=0.02). At two weeks following hemostatic agent use, a total of 17.2% patients encountered early or delayed BE, including 8.8% in the gel group vs 30.2% in the powder group (p=0.005). When compared to early BE, delayed BE required more frequent endoscopic re-interventions (87.5% vs 20%, p< 0.001).
Discussion: Regardless of indication, hemostatic gel was associated with lower bleeding rates compared to hemostatic powder, and delayed BE required more frequent endoscopic re-interventions. Ongoing prospective studies will seek to further validate these findings.

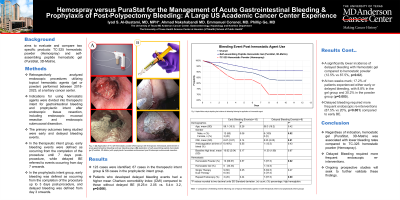
Figure: Figure 1: Kaplan-Meier analysis depicting the incidence of rebleeding following the application of a hemostatic agent.
Disclosures:
Iyad Al-Bustami indicated no relevant financial relationships.
Ahmad Nakshabandi indicated no relevant financial relationships.
Antonio Pizuorno Machado indicated no relevant financial relationships.
Daniel Low indicated no relevant financial relationships.
Emmanuel Coronel indicated no relevant financial relationships.
Phillip Ge: Alira Health – Consultant. Boston Scientific – Consultant. Neptune Medical – Consultant. Ovesco America – Consultant.
Iyad S.. Al-Bustami, MD1, Ahmad Nakshabandi, MD2, Antonio Pizuorno Machado, MD3, Daniel J. Low, MD4, Emmanuel Coronel, MD4, Phillip Ge, MD4. P3456 - Hemospray vs PuraStat for the Management of Acute Gastrointestinal Bleeding & Prophylaxis of Post-Polypectomy Bleeding: A Large US Academic Cancer Center Experience, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.

