Tuesday Poster Session
Category: IBD
P3531 - A Comparison of Weight Gain Profiles of Tumor Necrosis Factor (TNF)-Alpha Inhibitors, Ustekinumab, and Vedolizumab in the Treatment of Patients With Inflammatory Bowel Disease (IBD)
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
Has Audio
.jpg)
Navina Mohan, MD
NYU Langone Hospital-Brooklyn
Brooklyn, NY
Presenting Author(s)
Navina Mohan, MD1, Oliver Stewart, MD2, Arielle Leben, MS, RD, CDN3, Jordan Axelrad, MD, MPH4
1NYU Langone Hospital-Brooklyn, Brooklyn, NY; 2NYU Langone Hospital, New York, NY; 3NYU Langone Health Inflammatory Bowel Disease Center, New York, NY; 4NYU Grossman School of Medicine, New York, NY
Introduction: Inflammatory bowel disease (IBD), comprising Crohn’s disease and ulcerative colitis, is characterized by chronic inflammation of the gastrointestinal tract. IBD is known to contribute to micronutrient deficiencies, bone loss, weight loss, and in children, growth failure. TNF-alpha inhibitors have been implicated in weight gain in adults with rheumatologic conditions and children with IBD, but little is known about other IBD medical therapies. We aimed to compare the degree of weight change in IBD patients treated with TNF-alpha inhibitors, vedolizumab, or ustekinumab.
Methods: We performed a retrospective study of patients with IBD who initiated treatment with a TNF-alpha inhibitor (infliximab, adalimumab), vedolizumab, or ustekinumab between 2014-2019. Patient weight, in addition to measures of disease activity, was monitored at 0, 6, 12, 18, and 24-month intervals. Medication responders were defined as those who demonstrated a >3 point decrease in Harvey Bradshaw Index (HBI) or >1 point decrease in partial Mayo Score (pMayo). ANOVA testing was utilized to compare weight change between groups.
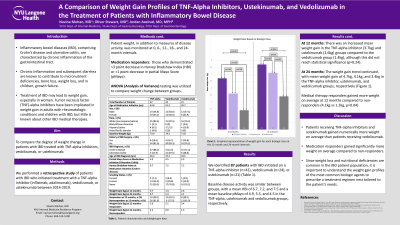
Results: We identified 87 patients with IBD initiated on a TNF-alpha inhibitor (n=42), vedolizumab (n=24), or ustekinumab (n=21) (Table 1). Baseline disease activity was similar between groups, with a mean HBI of 6.7, 7.2, and 7.5 and a mean baseline pMayo of 4.9, 5.5, and 4.5 in the TNF-alpha, ustekinumab, and vedolizumab groups, respectively. There was an increased mean weight gain in the TNF-alpha inhibitor (3.7kg) and ustekinumab (2.6kg) groups compared to the vedolizumab group (1.4kg) at 12 months, although this did not reach statistical significance (p=0.24). The weight gain trend continued at 24 months, with mean weight gain of 4.7kg, 4.1kg, and 2.4kg in the TNF-alpha inhibitor, ustekinumab, and vedolizumab groups, respectively (Figure 1). Medical therapy responders gained more weight on average at 12 months compared to non-responders (4.1kg vs 1.2kg, p=0.04).
Discussion: Patients receiving TNF-alpha inhibitors and ustekinumab gained numerically more weight on average than patients receiving vedolizumab. In addition, medication responders gained significantly more weight on average compared to non-responders. Given that weight loss and nutritional deficiencies are common in the IBD patient population, it is important to understand the weight gain profiles of the most common biologic agents.

Disclosures:
Navina Mohan, MD1, Oliver Stewart, MD2, Arielle Leben, MS, RD, CDN3, Jordan Axelrad, MD, MPH4. P3531 - A Comparison of Weight Gain Profiles of Tumor Necrosis Factor (TNF)-Alpha Inhibitors, Ustekinumab, and Vedolizumab in the Treatment of Patients With Inflammatory Bowel Disease (IBD), ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1NYU Langone Hospital-Brooklyn, Brooklyn, NY; 2NYU Langone Hospital, New York, NY; 3NYU Langone Health Inflammatory Bowel Disease Center, New York, NY; 4NYU Grossman School of Medicine, New York, NY
Introduction: Inflammatory bowel disease (IBD), comprising Crohn’s disease and ulcerative colitis, is characterized by chronic inflammation of the gastrointestinal tract. IBD is known to contribute to micronutrient deficiencies, bone loss, weight loss, and in children, growth failure. TNF-alpha inhibitors have been implicated in weight gain in adults with rheumatologic conditions and children with IBD, but little is known about other IBD medical therapies. We aimed to compare the degree of weight change in IBD patients treated with TNF-alpha inhibitors, vedolizumab, or ustekinumab.
Methods: We performed a retrospective study of patients with IBD who initiated treatment with a TNF-alpha inhibitor (infliximab, adalimumab), vedolizumab, or ustekinumab between 2014-2019. Patient weight, in addition to measures of disease activity, was monitored at 0, 6, 12, 18, and 24-month intervals. Medication responders were defined as those who demonstrated a >3 point decrease in Harvey Bradshaw Index (HBI) or >1 point decrease in partial Mayo Score (pMayo). ANOVA testing was utilized to compare weight change between groups.
Results: We identified 87 patients with IBD initiated on a TNF-alpha inhibitor (n=42), vedolizumab (n=24), or ustekinumab (n=21) (Table 1). Baseline disease activity was similar between groups, with a mean HBI of 6.7, 7.2, and 7.5 and a mean baseline pMayo of 4.9, 5.5, and 4.5 in the TNF-alpha, ustekinumab, and vedolizumab groups, respectively. There was an increased mean weight gain in the TNF-alpha inhibitor (3.7kg) and ustekinumab (2.6kg) groups compared to the vedolizumab group (1.4kg) at 12 months, although this did not reach statistical significance (p=0.24). The weight gain trend continued at 24 months, with mean weight gain of 4.7kg, 4.1kg, and 2.4kg in the TNF-alpha inhibitor, ustekinumab, and vedolizumab groups, respectively (Figure 1). Medical therapy responders gained more weight on average at 12 months compared to non-responders (4.1kg vs 1.2kg, p=0.04).
Discussion: Patients receiving TNF-alpha inhibitors and ustekinumab gained numerically more weight on average than patients receiving vedolizumab. In addition, medication responders gained significantly more weight on average compared to non-responders. Given that weight loss and nutritional deficiencies are common in the IBD patient population, it is important to understand the weight gain profiles of the most common biologic agents.

Figure: Figure 1: Comparing mean weight gain between 3 IBD medical therapy biologic classes at the 12 month and 24 month timeframes
Disclosures:
Navina Mohan indicated no relevant financial relationships.
Oliver Stewart indicated no relevant financial relationships.
Arielle Leben indicated no relevant financial relationships.
Jordan Axelrad: AbbVie – received consulting fees. Adiso – received consulting fees. BioFire Diagnostics – Consultant, Grant/Research Support. Bristol Myers Squibb – received consulting fees. Fresenius Kabi – received consulting fees. Janssen – received consulting fees. Pfizer – received consulting fees.
Navina Mohan, MD1, Oliver Stewart, MD2, Arielle Leben, MS, RD, CDN3, Jordan Axelrad, MD, MPH4. P3531 - A Comparison of Weight Gain Profiles of Tumor Necrosis Factor (TNF)-Alpha Inhibitors, Ustekinumab, and Vedolizumab in the Treatment of Patients With Inflammatory Bowel Disease (IBD), ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
