Tuesday Poster Session
Category: IBD
P3532 - Effectiveness and Safety of Risankizumab Induction Therapy in Crohn’s Disease: Prospective Real-World Experience in a Large Tertiary Center
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
Has Audio

Adar Zinger, MD, PhD
University of Chicago Medicine, Inflammatory Bowel Disease Center
Chicago, IL
Presenting Author(s)
Adar Zinger, MD, PhD1, David K.. Choi, PharmD1, Natalie K. Choi, BA1, Nicole M. Garcia, BA1, Russell D.. Cohen, MD1, Sushila Dalal, MD2, Noa Krugliak Cleveland, MD1, David T. Rubin, MD3
1University of Chicago Medicine, Inflammatory Bowel Disease Center, Chicago, IL; 2University of Chicago Medicine, Chicago, IL; 3Inflammatory Bowel Disease Center, University of Chicago Medicine, Chicago, IL
Introduction: Risankizumab was approved by the FDA in June 2022 for the treatment of moderately to severely active Crohn’s disease. We report the real-world experience with risankizumab in Crohn’s disease in a large tertiary inflammatory bowel disease (IBD) center.
Methods: This is a prospective analysis of clinical outcomes of patients with Crohn’s disease treated with risankizumab. Clinical evaluations occurred at weeks 0, 2, 4, 8 and 12 using the Harvey-Bradshaw Index (HBI), fecal calprotectin (FCP) and C-reactive protein (CRP). Efficacy analysis was performed for patients with active luminal disease at baseline, defined as HBI≥5 and/or FCP≥250µg/g, and/or documentation of ulcers in ileocolonoscopy, and/or evidence of active disease per imaging. Clinical remission was defined as HBI< 5. Clinical response was defined as ≥3-point decrease in HBI and/or HBI< 5. Adverse events were documented.
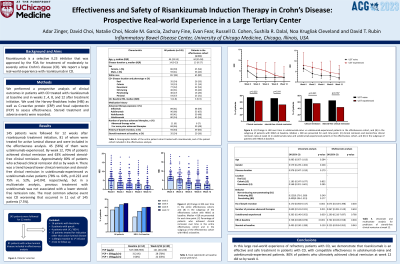
Results: 94 patients were followed for 12 weeks. Patients with ileostomy and patients that started treatment for indications other than active luminal disease were excluded from the effectiveness analysis. 54 patients met criteria to be assessed for treatment efficacy. Baseline characteristics are described in Table 1. Median HBI was noted to decrease by week 2 and plateaued by week 8 (Figure 1A). At week 12, 78% of patients achieved clinical response and 70% achieved clinical remission. This did not differ significantly between ustekinumab-naïve and ustekinumab-experienced patients (80% vs 76% p=0.72, 76% vs 65% p=0.11, respectively). Steroid-free clinical remission was higher in ustekinumab-naïve compared to ustekinumab-experienced patients (72% vs. 55%, p=0.003) (Figure 1B). 67% of patients (22/33) had CRP in the normal range at baseline, compared to 71% at week 12 (15/21). Median FCP decreased from 395 (IQR 329-940, n=9) at baseline, to 114 (IQR 0-163, n=25) at week 12. Minor adverse events were documented in 12% of patients, including fatigue, pruritus, nausea, joint pain, paresthesia, and upper-respiratory tract infection. One patient had a suspected allergic reaction to the infusion and treatment was stopped after one dose. No serious adverse events were observed.
Discussion: We demonstrate the clinical effectiveness and safety of risankizumab in a real-world tertiary population of patients with Crohn’s disease. The benefit was similar even in patients previously treated with ustekinumab.

Disclosures:
Adar Zinger, MD, PhD1, David K.. Choi, PharmD1, Natalie K. Choi, BA1, Nicole M. Garcia, BA1, Russell D.. Cohen, MD1, Sushila Dalal, MD2, Noa Krugliak Cleveland, MD1, David T. Rubin, MD3. P3532 - Effectiveness and Safety of Risankizumab Induction Therapy in Crohn’s Disease: Prospective Real-World Experience in a Large Tertiary Center, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1University of Chicago Medicine, Inflammatory Bowel Disease Center, Chicago, IL; 2University of Chicago Medicine, Chicago, IL; 3Inflammatory Bowel Disease Center, University of Chicago Medicine, Chicago, IL
Introduction: Risankizumab was approved by the FDA in June 2022 for the treatment of moderately to severely active Crohn’s disease. We report the real-world experience with risankizumab in Crohn’s disease in a large tertiary inflammatory bowel disease (IBD) center.
Methods: This is a prospective analysis of clinical outcomes of patients with Crohn’s disease treated with risankizumab. Clinical evaluations occurred at weeks 0, 2, 4, 8 and 12 using the Harvey-Bradshaw Index (HBI), fecal calprotectin (FCP) and C-reactive protein (CRP). Efficacy analysis was performed for patients with active luminal disease at baseline, defined as HBI≥5 and/or FCP≥250µg/g, and/or documentation of ulcers in ileocolonoscopy, and/or evidence of active disease per imaging. Clinical remission was defined as HBI< 5. Clinical response was defined as ≥3-point decrease in HBI and/or HBI< 5. Adverse events were documented.
Results: 94 patients were followed for 12 weeks. Patients with ileostomy and patients that started treatment for indications other than active luminal disease were excluded from the effectiveness analysis. 54 patients met criteria to be assessed for treatment efficacy. Baseline characteristics are described in Table 1. Median HBI was noted to decrease by week 2 and plateaued by week 8 (Figure 1A). At week 12, 78% of patients achieved clinical response and 70% achieved clinical remission. This did not differ significantly between ustekinumab-naïve and ustekinumab-experienced patients (80% vs 76% p=0.72, 76% vs 65% p=0.11, respectively). Steroid-free clinical remission was higher in ustekinumab-naïve compared to ustekinumab-experienced patients (72% vs. 55%, p=0.003) (Figure 1B). 67% of patients (22/33) had CRP in the normal range at baseline, compared to 71% at week 12 (15/21). Median FCP decreased from 395 (IQR 329-940, n=9) at baseline, to 114 (IQR 0-163, n=25) at week 12. Minor adverse events were documented in 12% of patients, including fatigue, pruritus, nausea, joint pain, paresthesia, and upper-respiratory tract infection. One patient had a suspected allergic reaction to the infusion and treatment was stopped after one dose. No serious adverse events were observed.
Discussion: We demonstrate the clinical effectiveness and safety of risankizumab in a real-world tertiary population of patients with Crohn’s disease. The benefit was similar even in patients previously treated with ustekinumab.

Figure: Figure 1. (A) HBI at weeks 0, 2, 4, 8, and 12. (B) Rates of clinical response, clinical remission, and steroid-free clinical remission at week 12 for ustekinumab naïve vs ustekinumab-experienced patients
Disclosures:
Adar Zinger indicated no relevant financial relationships.
David Choi: Abbvie – Consultant. Boehringer Ingelheim – Consultant. Bristol Myers Squibb – Consultant. Janssen Pharmaceuticals – Consultant, Speakers Bureau. Prometheus Laboratory – Consultant.
Natalie Choi indicated no relevant financial relationships.
Nicole Garcia indicated no relevant financial relationships.
Russell Cohen: AbbVie – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Boehringer Ingelheim – Grant/Research Support. Bristol-Meyers Squibb/Celgene – Advisor or Review Panel Member, Consultant, Grant/Research Support. Crohn's and Colitis Foundation of America – Grant/Research Support. Eli Lilly – Advisor or Review Panel Member, Consultant. Genentech – Advisor or Review Panel Member, Consultant, Grant/Research Support. Gilead Sciences – Advisor or Review Panel Member, Consultant, Grant/Research Support. Hoffman La-Roche – Advisor or Review Panel Member, Consultant. Hollister – Grant/Research Support. Janssen – Advisor or Review Panel Member, Consultant. Medimmune – Grant/Research Support. Mesoblast, Ltd. – Grant/Research Support. Osiris Therapeutics – Grant/Research Support. Pfizer – Advisor or Review Panel Member, Consultant, Grant/Research Support. Receptos – Grant/Research Support. RedHill BioPharma – Grant/Research Support. Sanofi-Aventis – Grant/Research Support. Schwarz Pharma – Grant/Research Support. Seres Therapeutics – Grant/Research Support. Takeda – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau.
Sushila Dalal: Abbvie – Speakers Bureau. Pfizer – Advisory Committee/Board Member.
Noa Krugliak Cleveland: Bristol Meyer Squibb – Speakers Bureau. Neurologica – Consultant. Takeda – Consultant.
David Rubin: AbbVie – Consultant, personal fees. AltruBio – Consultant, personal fees. Aslan Pharmaceuticals – Consultant. Athos Therapeutics – Consultant. Bellatrix Pharmaceuticals – Consultant. Boehringer Ingelheim – Consultant, personal fees. Bristol Myers Squibb – Consultant. Celgene Chronicles – Consultant. ClostraBio – Consultant. Connect BioPharma – Consultant. Corp/Syneos – Consultant. Eco R1 – Consultant. GastroIntestinal Research Foundation – Grant/Research Support. Genentech/Roche – Consultant. Gilead Sciences – Consultant, personal fees. Helmsley Charitable Trust – Grant/Research Support. Iterative Health – Consultant. Janssen Pharmaceuticals – Consultant, personal fees. Kaleido Biosciences – Consultant. Lilly – Consultant. Pfizer – Consultant, personal fees. Prometheus Biosciences – Consultant. Reistone Biopharma – Consultant, personal fees. Seres Therapeutics – Consultant. Takeda – Consultant, Grant/Research Support, Personal fees. Target RWE – Consultant. Trellus Health – Consultant.
Adar Zinger, MD, PhD1, David K.. Choi, PharmD1, Natalie K. Choi, BA1, Nicole M. Garcia, BA1, Russell D.. Cohen, MD1, Sushila Dalal, MD2, Noa Krugliak Cleveland, MD1, David T. Rubin, MD3. P3532 - Effectiveness and Safety of Risankizumab Induction Therapy in Crohn’s Disease: Prospective Real-World Experience in a Large Tertiary Center, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
