Tuesday Poster Session
Category: IBD
P3533 - Obefazimod in Patients With Moderate-to-Severe Ulcerative Colitis: Efficacy and Safety Analysis from the 96-week Open-label Maintenance Phase 2b Study
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
Has Audio
- SS
Sheldon Sloan, MD
Abivax
Paris, Ile-de-France, France
Presenting Author(s)
Séverine Vermeire, MD, PhD1, Josianne Nitcheu, PhD2, Paul Gineste, 2, Sheldon Sloan, MD2, Hartmut J. Ehrlich, MD2, Bruce E. Sands, MD, MS, FACG3
1UZ Leuven, Leuven, Vlaams-Brabant, Belgium; 2Abivax, Paris, Ile-de-France, France; 3Icahn School of Medicine at Mount Sinai, New York, NY
Introduction: Obefazimod is an oral small molecule that modulates inflammation by upregulating a specific anti-inflammatory micro-RNA (miR-124). Obefazimod has demonstrated safety and efficacy in patients with moderate-to-severe ulcerative colitis (UC) in the randomized, placebo-controlled, phase 2b induction study (1). This analysis presents data at week 96 after subjects enrolled in the open-label (OL) maintenance study.
Methods: Patients received placebo or obefazimod 25mg, 50mg or 100mg once daily (od) during the 16-week induction Phase 2b and, irrespective of their clinical response, could enter the optional OL 96-week maintenance study with obefazimod 50mg od. A total of 217 patients were enrolled into this study from January 13, 2020 onwards. Patients were followed monthly (first year) or every three months (2nd year) for safety and efficacy. Non-responder imputation (NRI) was used for missing efficacy data.
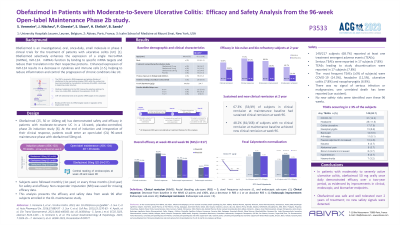
Results: Of 222 patients who completed the phase 2b induction study, 217 (97.7%) were enrolled in the OL maintenance study. 30/217 (13.8%) subjects dropped out prior to week 48, 6 subjects did not qualify the second year of treatment (non-responders) and 17/181 (9.4%) subjects dropped out between week 48 and week 96. All dropouts were considered as treatment failures for this analysis. 164 subjects completed the second year of treatment. Main efficacy results are presented in the Table below.
Among the 168 patients with no clinical remission after week 16 induction, 81 (48.2 %) achieved de novo clinical remission at week 96 of the OL maintenance study. Among the 49 patients with clinical remission after induction, 33 (67.3 %) maintained remission at week 96.
In total, 149 /217 subjects (68.7 %) reported at least one treatment emergent adverse events (TEAE) over 2 years of treatment. TEAEs leading to study discontinuation were reported in 17 subjects (7.8 %) and serious adverse events (SAEs) were reported in 18 subjects (8.3 %). The most frequent TEAEs (≥ 5 %) were COVID-19 (14.3 %), headache (11.5%), ulcerative colitis (7.8 %) and nasopharyngitis (6.9 %). No new safety risks were identified over these 96 weeks.
Discussion: Results from this 96-week analysis of the OL maintenance Phase 2b study confirmed long-term efficacy of obefazimod 50 mg od, with durable clinical remission, as well as a favorable long-term safety profile.
Reference:
1. Vermeire S, et al. Lancet Gastroenterol Hepatol 2022, 7 (11): 1024-1035
Disclosures:
Séverine Vermeire, MD, PhD1, Josianne Nitcheu, PhD2, Paul Gineste, 2, Sheldon Sloan, MD2, Hartmut J. Ehrlich, MD2, Bruce E. Sands, MD, MS, FACG3. P3533 - Obefazimod in Patients With Moderate-to-Severe Ulcerative Colitis: Efficacy and Safety Analysis from the 96-week Open-label Maintenance Phase 2b Study, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1UZ Leuven, Leuven, Vlaams-Brabant, Belgium; 2Abivax, Paris, Ile-de-France, France; 3Icahn School of Medicine at Mount Sinai, New York, NY
Introduction: Obefazimod is an oral small molecule that modulates inflammation by upregulating a specific anti-inflammatory micro-RNA (miR-124). Obefazimod has demonstrated safety and efficacy in patients with moderate-to-severe ulcerative colitis (UC) in the randomized, placebo-controlled, phase 2b induction study (1). This analysis presents data at week 96 after subjects enrolled in the open-label (OL) maintenance study.
Methods: Patients received placebo or obefazimod 25mg, 50mg or 100mg once daily (od) during the 16-week induction Phase 2b and, irrespective of their clinical response, could enter the optional OL 96-week maintenance study with obefazimod 50mg od. A total of 217 patients were enrolled into this study from January 13, 2020 onwards. Patients were followed monthly (first year) or every three months (2nd year) for safety and efficacy. Non-responder imputation (NRI) was used for missing efficacy data.
Results: Of 222 patients who completed the phase 2b induction study, 217 (97.7%) were enrolled in the OL maintenance study. 30/217 (13.8%) subjects dropped out prior to week 48, 6 subjects did not qualify the second year of treatment (non-responders) and 17/181 (9.4%) subjects dropped out between week 48 and week 96. All dropouts were considered as treatment failures for this analysis. 164 subjects completed the second year of treatment. Main efficacy results are presented in the Table below.
Among the 168 patients with no clinical remission after week 16 induction, 81 (48.2 %) achieved de novo clinical remission at week 96 of the OL maintenance study. Among the 49 patients with clinical remission after induction, 33 (67.3 %) maintained remission at week 96.
In total, 149 /217 subjects (68.7 %) reported at least one treatment emergent adverse events (TEAE) over 2 years of treatment. TEAEs leading to study discontinuation were reported in 17 subjects (7.8 %) and serious adverse events (SAEs) were reported in 18 subjects (8.3 %). The most frequent TEAEs (≥ 5 %) were COVID-19 (14.3 %), headache (11.5%), ulcerative colitis (7.8 %) and nasopharyngitis (6.9 %). No new safety risks were identified over these 96 weeks.
Discussion: Results from this 96-week analysis of the OL maintenance Phase 2b study confirmed long-term efficacy of obefazimod 50 mg od, with durable clinical remission, as well as a favorable long-term safety profile.
Reference:
1. Vermeire S, et al. Lancet Gastroenterol Hepatol 2022, 7 (11): 1024-1035
Disclosures:
Séverine Vermeire: AbbVie – Consultant, Grant/Research Support. AbolerIS Pharma – Grant/Research Support. AgomAb – Grant/Research Support. Alimentiv – Consultant. Arena Pharmaceuticals – Consultant. AstraZeneca – Consultant. Avaxia Biologics – Consultant. Boehringer Ingelheim – Consultant. Bristol Myers Squibb – Consultant. Celgene – Consultant. CVasThera – Consultant, Speakers Bureau. Cytoki Pharma – Consultant, Speakers Bureau. Dr. Falk Pharma – Consultant, Speakers Bureau. Eli Lilly – Consultant, Speakers Bureau. Ferring Pharmaceuticals – Consultant, Speakers Bureau. Galapagos – Consultant, Grant/Research Support, Speakers Bureau. Genentech/Roche – Consultant, Speakers Bureau. Gilead – Consultant, Speakers Bureau. GSK – Consultant, Speakers Bureau. Hospira – Consultant, Speakers Bureau. IMIDomics – Consultant, Speakers Bureau. Janssen Pharmaceuticals – Consultant, Speakers Bureau. Johnson & Johnson – Consultant, Grant/Research Support, Speakers Bureau. Materia Prima – Consultant, Speakers Bureau. MiroBio – Consultant, Speakers Bureau. Morphic – Consultant, Speakers Bureau. MRM Health – Consultant, Speakers Bureau. MSD – Consultant, Speakers Bureau. Mundipharma – Consultant, Speakers Bureau. Pfizer – Consultant, Grant/Research Support, Speakers Bureau. ProDigest – Consultant, Speakers Bureau. Progenity – Consultant, Speakers Bureau. Prometheus – Consultant, Speakers Bureau. Robarts Clinical Trials – Consultant, Speakers Bureau. Second Genome – Consultant, Speakers Bureau. Shire – Consultant, Speakers Bureau. Surrozen – Consultant, Speakers Bureau. Takeda – Consultant, Grant/Research Support, Speakers Bureau. Theravance Biopharma – Consultant, Speakers Bureau. Tillotts Pharma – Consultant, Speakers Bureau. Zealand Pharma – Consultant, Speakers Bureau.
Josianne Nitcheu: Abivax – Employee.
Paul Gineste: Abivax – Employee.
Sheldon Sloan: Abivax – Employee.
Hartmut Ehrlich: Abivax – Employee.
Bruce Sands: AbbVie – Consultant. Abivax – Consultant, Speaker’s fees. Adiso Therapeutics – Consultant. Alimentiv – Consultant. Amgen – Consultant. Arena pharmaceuticals – Consultant. Artizan Biosciences – Consultant. Artugen Therapeutics – Consultant. AstraZeneca – Consultant. Bacainn Therapeutics – Consultant. Biora Therapeutics – Consultant. Boehringer Ingelheim – Consultant. Boston Pharmaceuticals – Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, speaking fees and other support. Calibr – Consultant. Celltrion – Consultant. ClostraBio – Consultant. Connect Biopharm – Consultant. Cytoki Pharma – Consultant. Eli Lilly – Consultant, speaking fees and other support. Enthera – Consultant. Evommune – Consultant. Ferring – Consultant. Fresenius Kabi – Consultant. Galapagos – Consultant. Genentech – Consultant. Gilead Sciences – Consultant. GlaxoSmithKline – Consultant. Gossamer Bio – Consultant. HMP Acquisition – Consultant. Imhotex – Consultant. Immunic – Consultant. InDex Pharmaceuticals – Consultant. Innovation Therapeutics – Consultant. Inotrem – Consultant. Ironwood Pharmaceuticals – Consultant. Janssen – Grant/Research Support, consulting and speaking fees and other support. Johnson & Johnson – Consultant. Kaleido Biosciences – Consultant. Kallyope – Consultant. Merck – Consultant. MiroBio – Consultant. Morphic Therapeutics – Consultant. MRM Health – Consultant. OSE Immunotherapeutics – Consultant. Pfizer Inc – Consultant, Grant/Research Support, speaking fees and other support. Progenity – Consultant. Prometheus Biosciences – Consultant. Prometheus Laboratories – Consultant. Protagonist Therapeutics – Consultant. Q32 Bio – Consultant. RedHill Biopharma – Consultant. Sun Pharma – Consultant. Surrozen – Consultant. Synlogic Operating Company – Consultant. Takeda – Grant/Research Support, consulting and speaking fees and other support. Target RWE – Consultant. Theravance Biopharma – Consultant, Grant/Research Support. TLL Pharmaceutical – Consultant. USWM Enterprises – Consultant. Ventyx Biosciences – Consultant, personal fees and stock options for consulting. Viela Bio – Consultant.
Séverine Vermeire, MD, PhD1, Josianne Nitcheu, PhD2, Paul Gineste, 2, Sheldon Sloan, MD2, Hartmut J. Ehrlich, MD2, Bruce E. Sands, MD, MS, FACG3. P3533 - Obefazimod in Patients With Moderate-to-Severe Ulcerative Colitis: Efficacy and Safety Analysis from the 96-week Open-label Maintenance Phase 2b Study, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
