Tuesday Poster Session
Category: IBD
P3535 - Correlation of miR-124 Upregulation and PK Parameters in Blood of Patients With Moderate-to-Severe Ulcerative Colitis Receiving Obefazimod for 16 Weeks
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
Has Audio
- SS
Sheldon Sloan, MD
Abivax
Paris, Ile-de-France, France
Presenting Author(s)
Julien Santo, PhD1, Aurélien Flatres, PhD1, Paul Gineste, 2, Didier Scherrer, PhD1, Josianne Nitcheu, PhD2, Sheldon Sloan, MD2, Hartmut J. Ehrlich, MD2, Bruce E. Sands, MD, MS, FACG3, Séverine Vermeire, MD, PhD4
1Abivax, Montpellier, Languedoc-Roussillon, France; 2Abivax, Paris, Ile-de-France, France; 3Icahn School of Medicine at Mount Sinai, New York, NY; 4UZ Leuven, Leuven, Vlaams-Brabant, Belgium
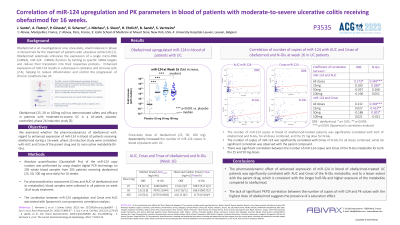
Introduction: Obefazimod is an oral small molecule that has demonstrated short- and long-term efficacy and good safety profile in patients with moderate-to-severe ulcerative colitis (UC) (1,2). Obefazimod modulates inflammation by upregulating a specific anti-inflammatory micro-RNA (miR-124). In humans, obefazimod is metabolized via UGT1A9 as a main active metabolite (N-Glu). We examined whether the pharmacodynamics of obefazimod with regard to miR-124 upregulation in blood of patients receiving obefazimod during a 16-week phase 2b induction study (EudraCT number: 2018-003558-26) were correlated with AUC and Cmax of the parent drug and its metabolite.
Methods: Absolute quantification (QuantaSoft Pro) of the miR-124 copy number was performed by using droplet digital PCR technology on 205 whole blood samples from 205 patients receiving obefazimod (25, 50, 100 mg once daily) for 16 weeks. For pharmacokinetics assessment (Cmax and AUC of obefazimod and its metabolite), blood samples were collected in all patients on week 16 of study treatment. The correlation between miR-124 upregulation and Cmax and AUC was tested with Spearman's non-parametric correlation analysis.
Results: Daily obefazimod treatment (25, 50, 100 mg) dose dependently increased the number of miR-124 copies in blood of UC patients (Table). miR-124 upregulation at week 16 in blood of obefazimod-treated patients was significantly correlated with AUC for obefazimod and its metabolite. The miR-124 upregulation showed a significant correlation with Cmax of N-Glu, while no significant correlation was observed with the parent compound. There was a dose-dependent correlation between miR-124 upregulation and Cmax of the metabolite.
Discussion: The pharmacodynamics of obefazimod with regard to miR-124 upregulation in blood of patients receiving obefazimod for 16 weeks correlates significantly with AUC and Cmax of N-Glu, and to a lesser extent with the parent drug. These findings are indicative of a dominant effect of N-Glu on miR-124 expression, which is consistent with the longer half-life and higher exposure of the metabolite compared to obefazimod. The lack of significant PKPD correlation between miR-124 increase and PK values with the highest dose of obefazimod suggests a saturation effect.
References:
1.Vermeire S, et al, Lancet Gastroenterol Hepatol 2022, 7 (11): 1024-1035
2. Vermeire S, et al, J Crohns Colitis 2023. doi: 10.1093/ecco-jcc/jjad067
Disclosures:
Julien Santo, PhD1, Aurélien Flatres, PhD1, Paul Gineste, 2, Didier Scherrer, PhD1, Josianne Nitcheu, PhD2, Sheldon Sloan, MD2, Hartmut J. Ehrlich, MD2, Bruce E. Sands, MD, MS, FACG3, Séverine Vermeire, MD, PhD4. P3535 - Correlation of miR-124 Upregulation and PK Parameters in Blood of Patients With Moderate-to-Severe Ulcerative Colitis Receiving Obefazimod for 16 Weeks, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Abivax, Montpellier, Languedoc-Roussillon, France; 2Abivax, Paris, Ile-de-France, France; 3Icahn School of Medicine at Mount Sinai, New York, NY; 4UZ Leuven, Leuven, Vlaams-Brabant, Belgium
Introduction: Obefazimod is an oral small molecule that has demonstrated short- and long-term efficacy and good safety profile in patients with moderate-to-severe ulcerative colitis (UC) (1,2). Obefazimod modulates inflammation by upregulating a specific anti-inflammatory micro-RNA (miR-124). In humans, obefazimod is metabolized via UGT1A9 as a main active metabolite (N-Glu). We examined whether the pharmacodynamics of obefazimod with regard to miR-124 upregulation in blood of patients receiving obefazimod during a 16-week phase 2b induction study (EudraCT number: 2018-003558-26) were correlated with AUC and Cmax of the parent drug and its metabolite.
Methods: Absolute quantification (QuantaSoft Pro) of the miR-124 copy number was performed by using droplet digital PCR technology on 205 whole blood samples from 205 patients receiving obefazimod (25, 50, 100 mg once daily) for 16 weeks. For pharmacokinetics assessment (Cmax and AUC of obefazimod and its metabolite), blood samples were collected in all patients on week 16 of study treatment. The correlation between miR-124 upregulation and Cmax and AUC was tested with Spearman's non-parametric correlation analysis.
Results: Daily obefazimod treatment (25, 50, 100 mg) dose dependently increased the number of miR-124 copies in blood of UC patients (Table). miR-124 upregulation at week 16 in blood of obefazimod-treated patients was significantly correlated with AUC for obefazimod and its metabolite. The miR-124 upregulation showed a significant correlation with Cmax of N-Glu, while no significant correlation was observed with the parent compound. There was a dose-dependent correlation between miR-124 upregulation and Cmax of the metabolite.
Discussion: The pharmacodynamics of obefazimod with regard to miR-124 upregulation in blood of patients receiving obefazimod for 16 weeks correlates significantly with AUC and Cmax of N-Glu, and to a lesser extent with the parent drug. These findings are indicative of a dominant effect of N-Glu on miR-124 expression, which is consistent with the longer half-life and higher exposure of the metabolite compared to obefazimod. The lack of significant PKPD correlation between miR-124 increase and PK values with the highest dose of obefazimod suggests a saturation effect.
References:
1.Vermeire S, et al, Lancet Gastroenterol Hepatol 2022, 7 (11): 1024-1035
2. Vermeire S, et al, J Crohns Colitis 2023. doi: 10.1093/ecco-jcc/jjad067
Disclosures:
Julien Santo: Abivax – Employee.
Aurélien Flatres: Abivax – Employee.
Paul Gineste: Abivax – Employee.
Didier Scherrer: Abivax – Employee.
Josianne Nitcheu: Abivax – Employee.
Sheldon Sloan: Abivax – Employee.
Hartmut Ehrlich: Abivax – Employee.
Bruce Sands: AbbVie – Consultant. Abivax – Consultant, Speaker’s fees. Adiso Therapeutics – Consultant. Alimentiv – Consultant. Amgen – Consultant. Arena pharmaceuticals – Consultant. Artizan Biosciences – Consultant. Artugen Therapeutics – Consultant. AstraZeneca – Consultant. Bacainn Therapeutics – Consultant. Biora Therapeutics – Consultant. Boehringer Ingelheim – Consultant. Boston Pharmaceuticals – Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, speaking fees and other support. Calibr – Consultant. Celltrion – Consultant. ClostraBio – Consultant. Connect Biopharm – Consultant. Cytoki Pharma – Consultant. Eli Lilly – Consultant, speaking fees and other support. Enthera – Consultant. Evommune – Consultant. Ferring – Consultant. Fresenius Kabi – Consultant. Galapagos – Consultant. Genentech – Consultant. Gilead Sciences – Consultant. GlaxoSmithKline – Consultant. Gossamer Bio – Consultant. HMP Acquisition – Consultant. Imhotex – Consultant. Immunic – Consultant. InDex Pharmaceuticals – Consultant. Innovation Therapeutics – Consultant. Inotrem – Consultant. Ironwood Pharmaceuticals – Consultant. Janssen – Grant/Research Support, consulting and speaking fees and other support. Johnson & Johnson – Consultant. Kaleido Biosciences – Consultant. Kallyope – Consultant. Merck – Consultant. MiroBio – Consultant. Morphic Therapeutics – Consultant. MRM Health – Consultant. OSE Immunotherapeutics – Consultant. Pfizer Inc – Consultant, Grant/Research Support, speaking fees and other support. Progenity – Consultant. Prometheus Biosciences – Consultant. Prometheus Laboratories – Consultant. Protagonist Therapeutics – Consultant. Q32 Bio – Consultant. RedHill Biopharma – Consultant. Sun Pharma – Consultant. Surrozen – Consultant. Synlogic Operating Company – Consultant. Takeda – Grant/Research Support, consulting and speaking fees and other support. Target RWE – Consultant. Theravance Biopharma – Consultant, Grant/Research Support. TLL Pharmaceutical – Consultant. USWM Enterprises – Consultant. Ventyx Biosciences – Consultant, personal fees and stock options for consulting. Viela Bio – Consultant.
Séverine Vermeire: AbbVie – Consultant, Grant/Research Support. AbolerIS Pharma – Grant/Research Support. AgomAb – Grant/Research Support. Alimentiv – Consultant. Arena Pharmaceuticals – Consultant. AstraZeneca – Consultant. Avaxia Biologics – Consultant. Boehringer Ingelheim – Consultant. Bristol Myers Squibb – Consultant. Celgene – Consultant. CVasThera – Consultant, Speakers Bureau. Cytoki Pharma – Consultant, Speakers Bureau. Dr. Falk Pharma – Consultant, Speakers Bureau. Eli Lilly – Consultant, Speakers Bureau. Ferring Pharmaceuticals – Consultant, Speakers Bureau. Galapagos – Consultant, Grant/Research Support, Speakers Bureau. Genentech/Roche – Consultant, Speakers Bureau. Gilead – Consultant, Speakers Bureau. GSK – Consultant, Speakers Bureau. Hospira – Consultant, Speakers Bureau. IMIDomics – Consultant, Speakers Bureau. Janssen Pharmaceuticals – Consultant, Speakers Bureau. Johnson & Johnson – Consultant, Grant/Research Support, Speakers Bureau. Materia Prima – Consultant, Speakers Bureau. MiroBio – Consultant, Speakers Bureau. Morphic – Consultant, Speakers Bureau. MRM Health – Consultant, Speakers Bureau. MSD – Consultant, Speakers Bureau. Mundipharma – Consultant, Speakers Bureau. Pfizer – Consultant, Grant/Research Support, Speakers Bureau. ProDigest – Consultant, Speakers Bureau. Progenity – Consultant, Speakers Bureau. Prometheus – Consultant, Speakers Bureau. Robarts Clinical Trials – Consultant, Speakers Bureau. Second Genome – Consultant, Speakers Bureau. Shire – Consultant, Speakers Bureau. Surrozen – Consultant, Speakers Bureau. Takeda – Consultant, Grant/Research Support, Speakers Bureau. Theravance Biopharma – Consultant, Speakers Bureau. Tillotts Pharma – Consultant, Speakers Bureau. Zealand Pharma – Consultant, Speakers Bureau.
Julien Santo, PhD1, Aurélien Flatres, PhD1, Paul Gineste, 2, Didier Scherrer, PhD1, Josianne Nitcheu, PhD2, Sheldon Sloan, MD2, Hartmut J. Ehrlich, MD2, Bruce E. Sands, MD, MS, FACG3, Séverine Vermeire, MD, PhD4. P3535 - Correlation of miR-124 Upregulation and PK Parameters in Blood of Patients With Moderate-to-Severe Ulcerative Colitis Receiving Obefazimod for 16 Weeks, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
